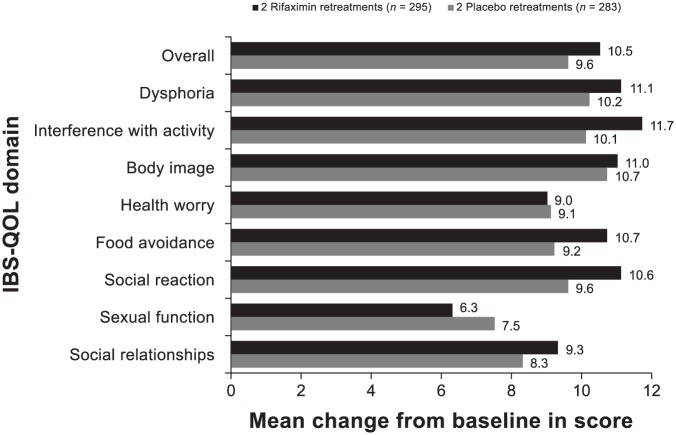
Figure 5.
Change from double-blind baseline in IBS-QOL overall and subdomain scores 6 weeks after receiving the first double-blind repeat treatment for patients receiving two repeat treatments with rifaximin or placebo in the double-blind phase. Analysis was performed using per protocol population. Positive numbers indicate an improvement from baseline in IBS-QOL score. Number of patients with missing data: rifaximin (n = 3) and placebo (n = 2), overall and all subdomain scores.
IBS-QOL, irritable bowel syndrome quality of life questionnaire.