Figure 2.
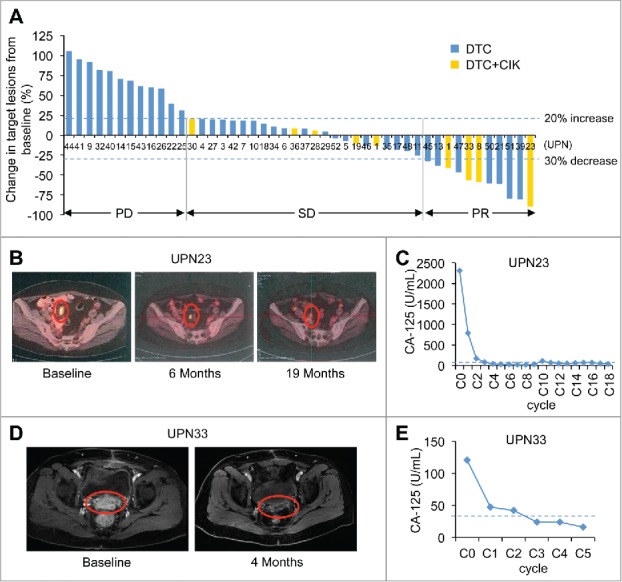
(A) Maximum change by RECIST (version 1.1) in ovarian cancer patients from baseline. Changes in target lesions from baseline in 46 patients with measurable disease who received DTC or DTC+CIK treatment. Each bar represents an individual patient in the clinical trial. The two horizontal dashed lines mark the thresholds for objective response (lower line, −30%) and PD (higher line, 20%), according to RECIST (version 1.1). (B) Partial response of platinum-resistant/refractory and recurrent high-grade serous ovarian cancer in a 56-y-old patient (UPN23), who received DTC+CIK treatment of 19 cycles. This patient had previously undergone primary surgery, and progressive disease had developed after treatment with systemic chemotherapies (i.e., cisplatin and docetaxel). The pelvic mesenteric lymph node metastases were observed on a baseline PET-CT image (left), these lesions were reduced at 6 mo (middle) and maintained for 19 mo (right) after the start of this treatment. The red circles show regression of recurrent lymph node metastases. (C) Tumor marker CA-125 decreased to normal range after five course of DAC-based therapy in patient UPN23. C0, baseline; C2, day 28 in cycle 2. The horizontal dashed line represents the cut-off level for CA-125 (35 U/mL). (D) Partial response of platinum-resistant and recurrent high-grade serous ovarian cancer in a 52-year-old patient (UPN33), who received DAC+TC+CIK treatment of five cycles, and PR maintained for 8 mo until disease progressed. The tumor lesions reduced after four cycles of treatment (right) as compared with the baseline (left) as analyzed by the MRI examination. (E) In UPN33, CA-125 level decreased to normal range after two cycles. C0, baseline; C1, day 28 in cycle 1. The horizontal dashed line represents the cut-off level for CA-125 (35 U/mL).
