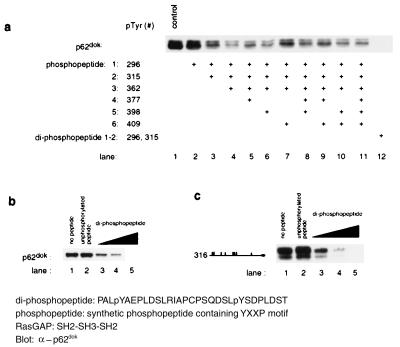
Figure 6.
Peptide competition analysis indicates that Tyr-296 and Tyr-315 play a critical role in the binding of p62dok to RasGAP. The ability of the diphosphopeptide to inhibit binding of Dok-1 to GAP suggests that the proper positioning of pTyr-296 and pTyr-315 in tandem is critical for the interaction of the two molecules. (a) Combinations of phosphopeptides corresponding to the regions surrounding individual tyrosines in p62dok fail to inhibit the binding of p62dok to RasGAP. However, a diphosphopeptide corresponding to residues 293–322 is able to inhibit binding of Dok-1 to the GAP SH2-SH3-SH2 region. Binding analysis was conducted as described. Synthetic phosphopeptides used for this experiment were: 1, SPPALpYAEPLDS (pTyr-296); 2, SQDSLpYSDPLDS (pTyr-315); 3, PKEDPIpYDEPEGL (pTyr-362); 4, VPPQG LpYDLPREPK (pTyr-377); 5, RVKEEGpYELPYNPATDD (pTyr-398); 6, NPATDD pYAVPPPR (pTyr-409); and diphosphopeptide, PALpYAEPLDSLRIAPCPSQDS LpYSDPLDST (pTyr-296 and pTyr-315). For control, unphosphorylated peptides were used. Each phosphopeptide was added in concentration of 50 μM. (b) Dose-dependent inhibition of p62dok binding to RasGAP by diphosphopeptide (Dok-1 aa 293–322). The diphosphopeptide was added in concentration of 0.5, 5, or 50 μM. The unphosphorylated peptide was added in concentration of 50 μM. (c) Dose-dependent inhibition of a truncated Dok-1 (the truncation construct 316: residues 316–481) binding to RasGAP by diphosphopeptide. The diphosphopeptide was added in concentration of 0.5, 5, or 50 μM. The unphosphorylated peptide was added in concentration of 50 μM.