Figure 5. S-acylation of EGFP-Snap25b by DHHC17 and effect of DHHC17 mutations.
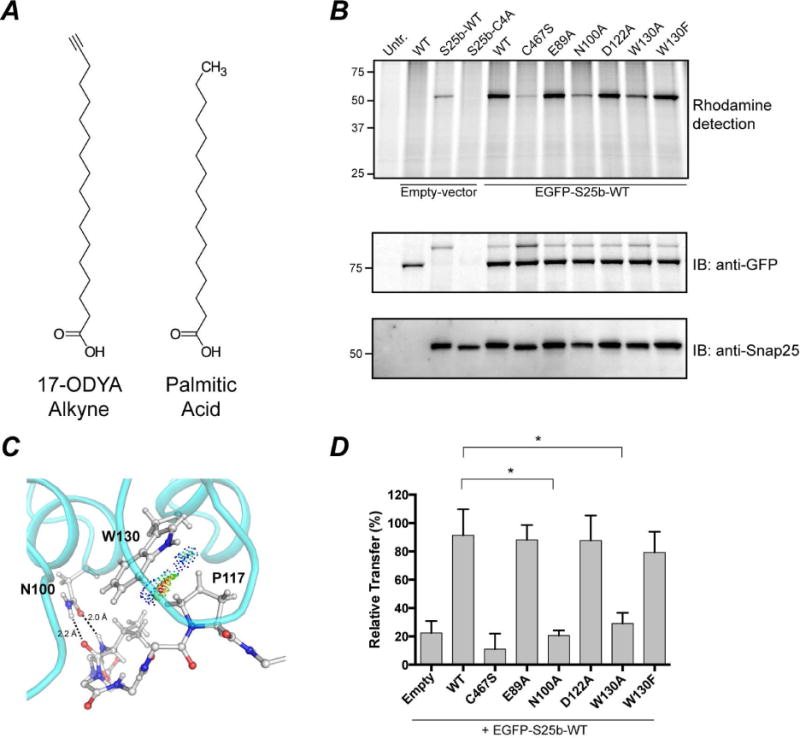
HEK-293T cells were transfected with EGFP-Snap25b, EGFP-Snap25b-C4A (cysteine-less) or empty vector and GFP-DHHC17 or GFP-DHHC17 mutants. Cells were incubated with 17-ODYA fatty acid alkyne for 4 h at 37°C. S-acylation with fatty acid alkyne was detected by coupling the alkyne fatty acid to Rhodamine-azide dye via click chemistry reaction. Isolated proteins were resolved by SDS/PAGE and transferred to PVDF membranes. (A) Chemical structures of 17-ODYA (17-Octadecynoic Acid) and palmitic acid. (B) Representative image of a typical experiment is shown. Click chemistry signal (Top), anti-GFP immunoblot (Middle), and anti-Snap25 immunoblot (Bottom). (C) van der Waals contacts between W130 and P117. Contact dots were obtained using the method of Richardson and co. (Word et al., 1999). (D) Quantification of Snap25b S-acylation. The intensity of the Snap25b band in the immunoblot was divided by the intensity of the Snap25b band in the rhodamine detection gel. Histograms represent average ± standard deviation (n=3). One-way ANOVA with Dunnett’s multiple comparison test was used to compare mutants with WT (n.s., p ≥ 0.05, *p < 0.05).
