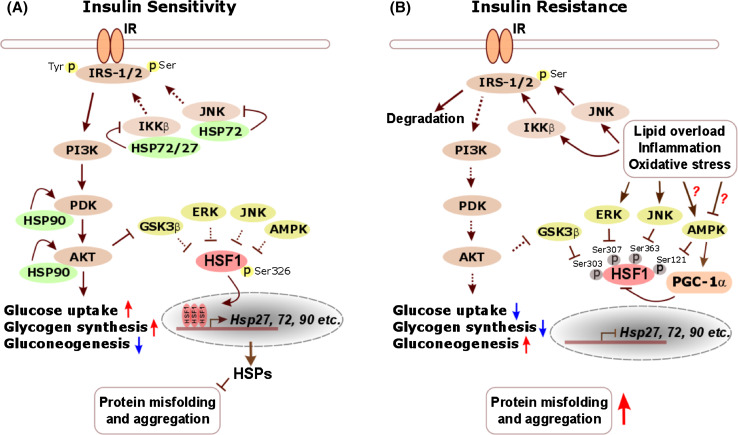
Fig. 2.
The HSF1-mediated PSR antagonizes insulin resistance and preserves proteostasis. a In insulin-sensitive cells, insulin signaling mobilizes AKT, which subsequently leads to inactivation of GSK3β [196], a negative regulator of HSF1. In addition, other HSF1 suppressors, including ERK, JNK and AMPK, also remain at a normal or latent state. As a consequence, the HSF1-mediated PSR is operational to induce abundant HSP expression. Importantly, HSP27 and HSP72 suppress both IKKβ and JNK [197–199], two key kinases that inhibit insulin receptor substrate-1/2 (IRS-1/2) through direct serine phosphorylation and thereby impede insulin signaling [200]. Moreover, HSP90 can stabilize and enhance the activation of both PDK and AKT [201, 202], two essential components within the insulin signaling cascade. Thus, in addition to preserving cellular proteostasis, the HSF1-mediated PSR maintains robust insulin signaling, enhancing glucose uptake and glycogen synthesis but suppressing gluconeogenesis. b In insulin-resistant cells, activated IKKβ and JNK, owing to lipid overload, inflammation, and oxidative stress, markedly phosphorylate IRS-1/2, causing their dissociation from IR and proteasomal degradation [203, 204]. Impaired insulin signaling further leads to enhanced GSK3β activity. In addition, inflammation and oxidative stress closely associated with the insulin-resistant state also activate ERK and JNK. However, it still remains controversial whether AMPK is activated under insulin resistance. Whereas lipid overload can suppress AMPK [205], oxidative stress is reported to cause its activation [206]. Collectively, these negative regulators deactivate HSF1 and its mediated PSR, depleting cellular HSPs to further exacerbate the impairment of insulin signaling and disruption of proteostasis