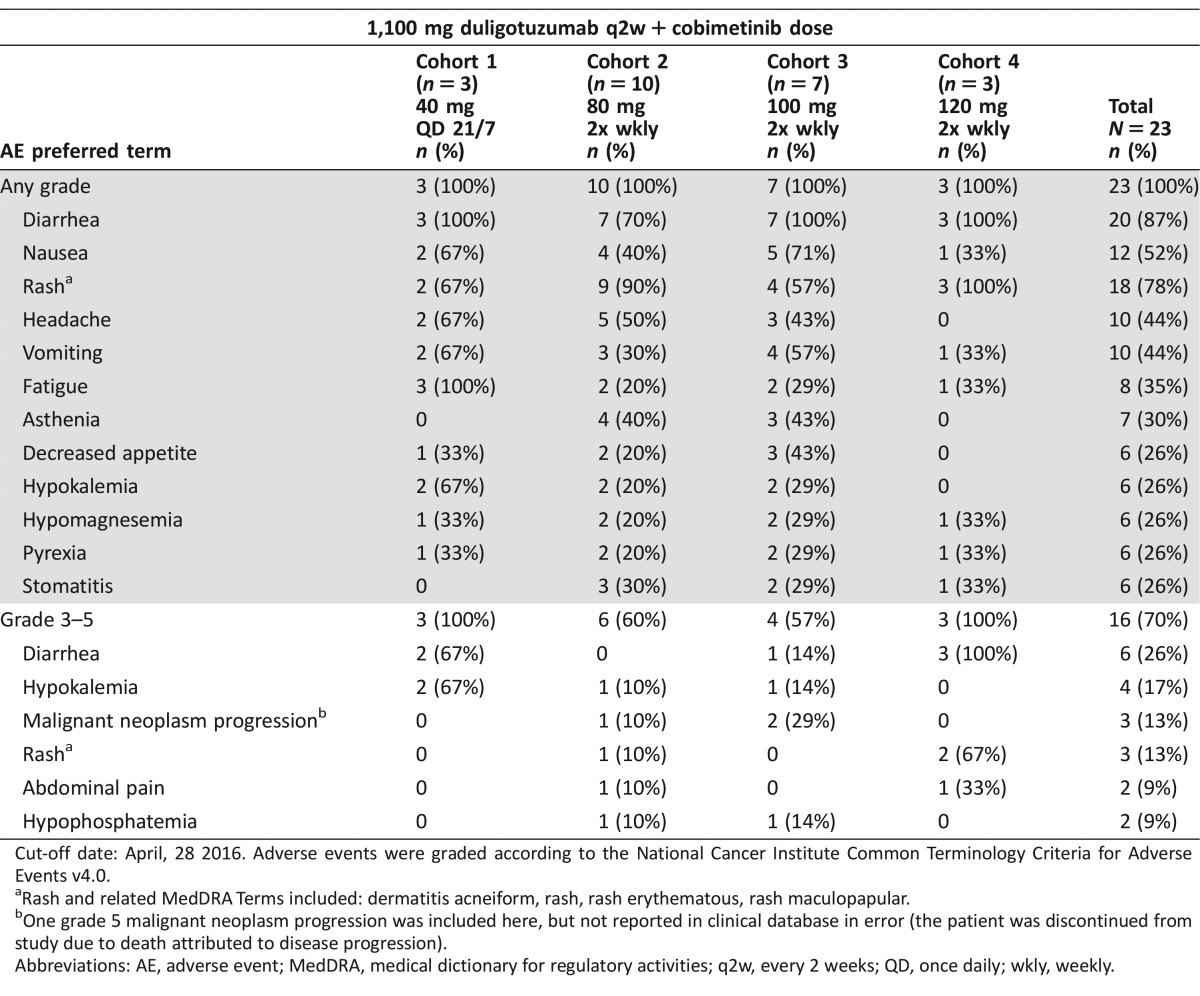
Table 1. All AEs in >25% patients regardless of causality, and all grade 3–4 AEs in ≥ 2 patients.
Cut‐off date: April, 28 2016. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.
Rash and related MedDRA Terms included: dermatitis acneiform, rash, rash erythematous, rash maculopapular.
One grade 5 malignant neoplasm progression was included here, but not reported in clinical database in error (the patient was discontinued from study due to death attributed to disease progression).
Abbreviations: AE, adverse event; MedDRA, medical dictionary for regulatory activities; q2w, every 2 weeks; QD, once daily; wkly, weekly.