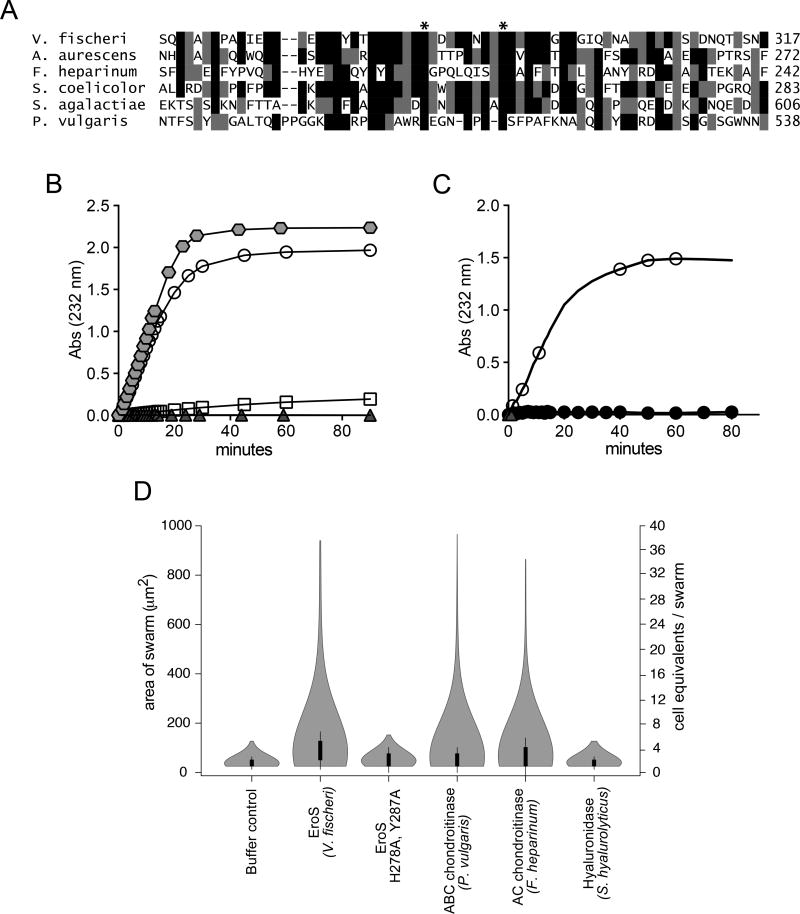
Figure 3. The V. fischeri aphrodisiac is a chondroitinase.
(A) Alignment of the V. fischeri EroS amino acid sequence to diverse bacterial GAG lyases reveals that V. fischeri harbors conserved His and Tyr residues (indicated by *) at sites required for catalytic activity in characterized GAG lyases (Han et al., 2014; Linhardt et al., 2006). Amino acids with >50% conservation between sequences are shaded (black shading for identical amino acids and grey shading for similar amino acids. (B) Purified EroS degrades chondroitin sulfate and hyaluronan. EroS was incubated with purified chondroitin sulfate (open circle), hyaluronan (grey hexagon), dermatan sulfate (open square), and heparan sulfate (grey triangle), and GAG lyase activity of EroS was measured by monitoring the abundance of unsaturated oligosaccharide reaction products with an absorbance at 232nm. Chondroitin sulfate and hyaluronan oligosaccharides accumulated rapidly in the presence of EroS, indicating depolymerization, whereas heparan sulfate and dermatan sulfate were not depolymerized by EroS. (C) Alanine substitutions at two predicted catalytic residues in EroS (H278 and Y287) eliminated the protein’s ability to degrade chondroitin sulfate. The chondroitinase activity of either wild type EroS (open circle) or EroS-H278A,Y287A (filled circle) against purified chondroitin sulfate was measured by monitoring the abundance of unsaturated oligosaccharide products with an absorbance at 232nm. (D) The chondroitinase activity of EroS is necessary and sufficient for its function as an aphrodisiac. EroS-H278A,Y287A failed to induce swarming in S. rosetta. P. vulgaris ABC chondroitinase and F. heparinum AC chondroitinase were sufficient to induce swarming at levels similar to EroS, whereas S. hyalurolyticus hyaluronidase failed to induce swarming, indicating that chondroitinase activity is necessary and sufficient for aphrodisiac activity.