Figure 2. RNA, protein and cell cycle studies in Subject 3 lymphoblasts encompassing a heterozygous ACTL6A splicing variant.
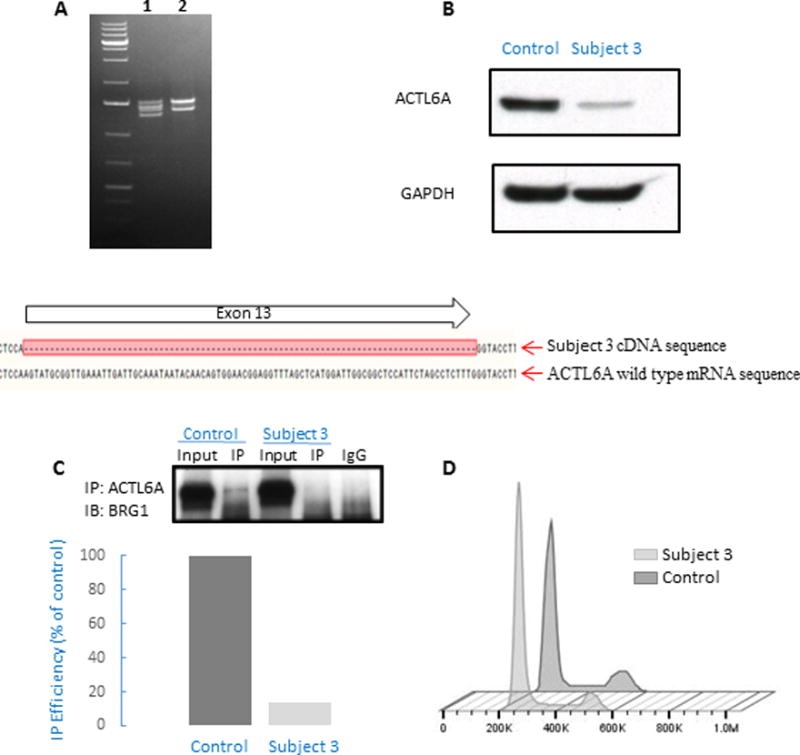
(A) RT-PCR amplification of the full-length cDNA from control sample (2) shows two transcripts due to alternative splicing at ACTL6A exon 1, while amplification of Subject 3 cDNA (1) shows four transcripts. PCR-cloning and sequencing, followed by alignment of the sequence results with ACTL6A mRNA sequence demonstrates that two of the four transcripts in Subject 3 correspond to expression of the two isoforms of wild-type allele, and two transcripts correspond to expression of the two isoforms of mutated allele containing an in-frame deletion of the full length of exon 13. (B) Western blot analysis of ACTL6A protein expression in Subject 3 and control cells, revealing reduced protein expression in Subject 3 cells that is suggestive of a haploinsufficiency mechanism.
(C) Immunoprecipitation (IP) with anti-ACTL6A antibody, showing decreased interaction with BRG1 in subject cells (Subject 3) compared to unrelated healthy control (Control). There was no binding to negative control (rabbit IgG). Immunoblot (IB) was quantified (using ImageJ program), showing 85% reduction in co-IP efficiency, presented here as % of control. The reduction of co-IP efficiency was repeated in two other independent experiments (showing 70% and 20% reduction, respectively). (D) Cell cycle analysis of cells from Subject 3 and control cells. As compared to control, a smaller fraction of cells from subject 3 resided in G2 phase (10.5 % vs. 16.8 % in control samples). This trend was observed in two independent experiments and is consistent with previous reports describing cell cycle perturbation in ACTL6A-depleted cells (Lee, et al., 2007).
