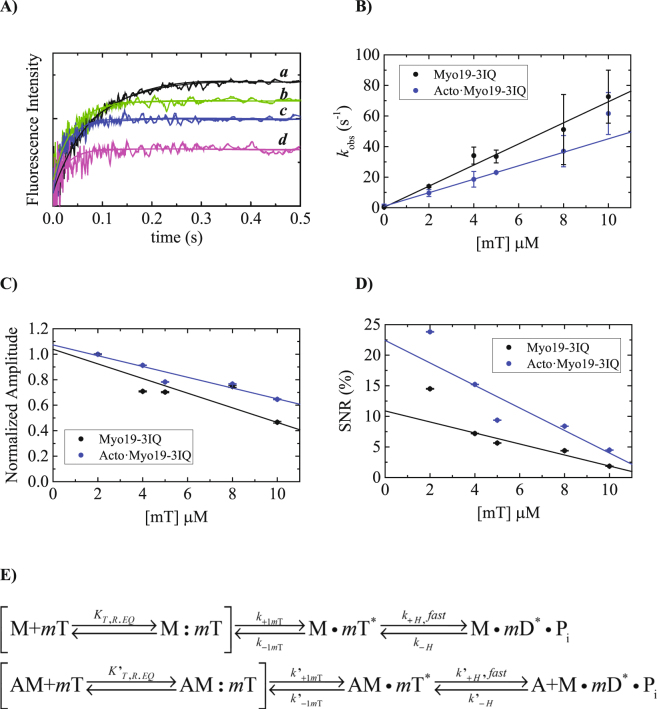
Figure 4.
Binding kinetics of mT to Myo19-3IQ and Acto·Myo19-3IQ by monitoring FRET upon tryptophan excitation to mant-fluorophore. (A) Time courses of mT fluorescence change after mixing 200 nM myosin 19-3IQ with 2 (a), 4, (b), 5 (c), 10 µM (d) mT (final concentrations). The smooth lines are the best fit to a single exponential. (B) The dependence of k obs on [mT]. The solid line through the data points is the best fits to linear equation. Error bars represent standard deviation from three independent experiments. (C) The dependence of the amplitudes on [mT]. The solid lines through the data points are the best fits to linear equation. Error bars of the fitting are within the data points. (D) The dependence of signal to noise ratio (SNR) of the experimental data on [mT]. Note the fast decrease in observable signals. (F) The kinetic reaction mechanism for mT binding to Myo19-3IQ and for Acto·Myo19-3IQ. We included in rectangular parenthesis a rapid collision complex that most likely occurs without fluorescence change.