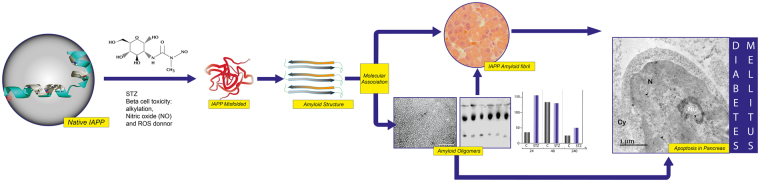
Figure 5.
The proposed mechanism of rIAPP aggregation/oligomerization as determined in STZ-induced diabetes in rats. The STZ produced cell stress, increasing protein aggregation and decreasing the capacity for proteostasis. rIAPP undergoes structural perturbations and enters an aggregation-competent state (misfolded), which may even contain β-breakers. The misfolded rIAPP takes on amyloidogenic features, leading to self- and hetero-oligomerization and, finally, conversion into rIAPP fibres. The cytotoxic oligomers cause apoptosis in several cells and the appearance of cross-seeding amyloid structures.