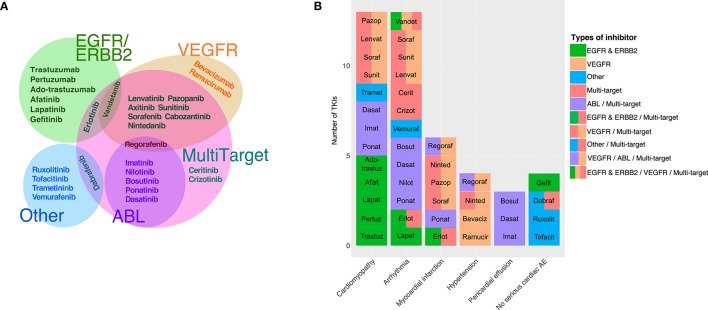
Figure 1.
TKI targets and associated adverse events. (A) Euler diagram of tyrosine kinase inhibitors grouped based on the primary intended target(s). The three major primary targets are EGFR/ERBB2 (8 TKIs), VEGFR (11 TKIs), and ABL (6 TKIs). The category “Other” comprises five relatively newer TKIs with primary targets in different categories, such as vemurafenib (B-Raf). Out of 30 approved TKIs, 18 were identified as having intended targets in more than one category. (B) Black box warnings associated with tyrosine kinase inhibitors are indicated, with closely-related toxicities grouped to ease visualization. Cardiomyopathy category includes: “cardiac dysfunction,” “congestive heart failure,” “left ventricular dysfunction,” and “cardiomyopathy.” Arrhythmia includes: “prolonged QT interval,” “cardiac bradyarrhythmia,” and “cardiac arrhythmia.” Pericardial effusion includes both “pericardial/pleural effusion,” and “cardiac tamponade.” Four approved drugs have no cardiac-associated boxed warning (i.e., no serious cardiac adverse events listed in the drug's package insert).