Figure 103.
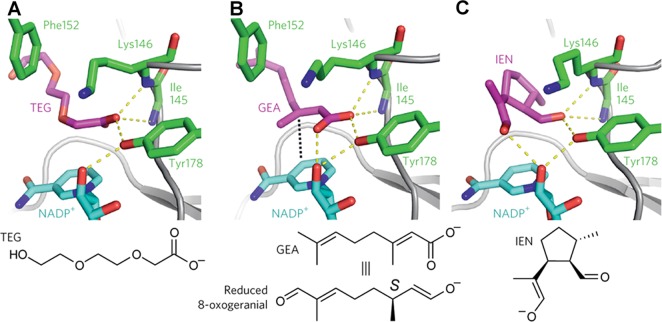
(A) The binding of triethylene glycol carboxylate (TEG) in the active site of the iridoid synthase-NADP+ complex reveals that a carboxylate oxygen receives hydrogen bonds from the backbone NH groups of I145 and K146 and the hydroxyl group of Y178. These interactions define an oxyanion hole in the active site. (B) The binding of the transition state analogue geranic acid (GEA) in the iridoid synthase–NADP+–GEA ternary complex reveals that its carboxylate oxygen similarly receives 3 hydrogen bonds in the oxyanion hole. These interactions likely stabilize the enolate oxyanion of reduced 8-oxogeranial formed by Michael addition of the pro-S NADPH hydride to C3 of the substrate (hydride trajectory indicated by a black dotted line). (C) Model of iridoid synthase complexed with the enolate form of iridodial (IEN) indicates that the enolate oxyanion can remain stabilized in the oxyanion hole; however, cyclization of the extended conformation represented by the binding of GEA in (B) requires a conformational change of F152, which would otherwise sterically block iridodial formation. Reprinted with permission from ref (538). Copyright 2016 Macmillan Publishers Ltd.