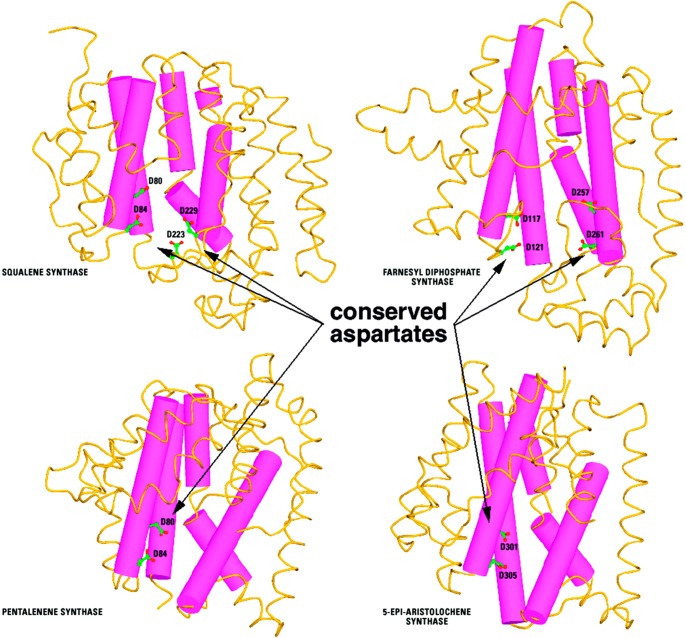
Figure 16.
Human squalene synthase, avian FPP synthase, bacterial pentalenene synthase, and plant 5-epi-aristolochene synthase share insignificant overall amino acid sequence identity, yet these enzymes share the class I terpenoid synthase fold with conservation of aspartate-rich metal-binding motifs as well as a kink in helix G (the purple helix bent into two parts connected by a short loop). This kink orients main chain carbonyl groups of helix G1 into the active site, where the negative electrostatic potential of the helix dipole may stabilize carbocation intermediates in isoprenoid coupling or cyclization reactions. Originally published in ref (107). Copyright 2000 The American Society for Biochemistry & Molecular Biology.