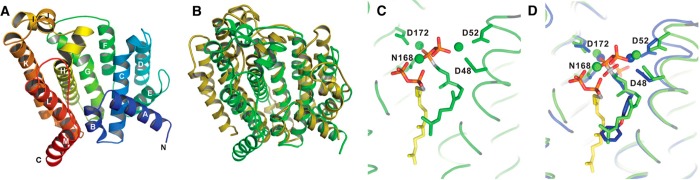
Figure 17.
(A) Dehydrosqualene synthase (CrtM) from S. aureus adopts the α fold of a class I terpenoid synthase. (B) Superposition of bacterial dehydrosqualene synthase (green) and human squalene synthase (yellow) reveals homologous structures. (C) Two molecules of the unreactive substrate analogue FSPP bind in the active site of dehydrosqualene synthase; one FSPP molecule is poised for ionization and allylic cation formation by coordination to 3 Mg2+ ions. (D) Crystal structure of dehydrosqualene synthase complexed with the inhibitor BPH-652 superimposed on the complex with two FSPP molecules reveals that the inhibitor binding site partially overlaps with both FSPP binding sites. Reprinted with permission from ref (109). Copyright 2008 AAAS.