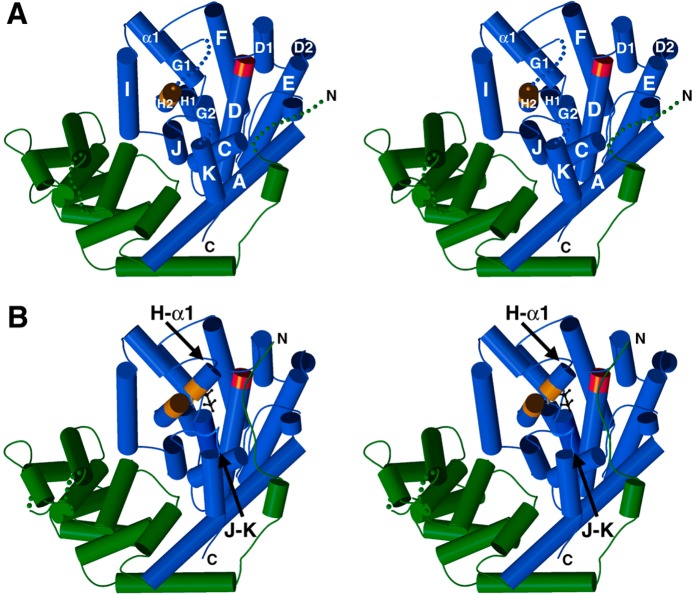
Figure 33.
(A) Stereoview of unliganded (+)-bornyl diphosphate synthase, looking into the active site in the α domain (blue). Disordered polypeptide segments are indicated by dotted lines and include the N-terminal segment of the β domain (green). Aspartate-rich and DTE metal-binding motifs are red and orange, respectively. (B) Stereoview of the (+)-bornyl diphosphate synthase-Mg2+3-inorganic pyrophosphate complex. Comparison with the unliganded structure in (A) reveals conformational changes that completely enclose the active site. These conformational changes include the ordering of the N-terminal segment, which helps cap the active site. Reprinted from ref (23). Copyright 2002 National Academy of Sciences.