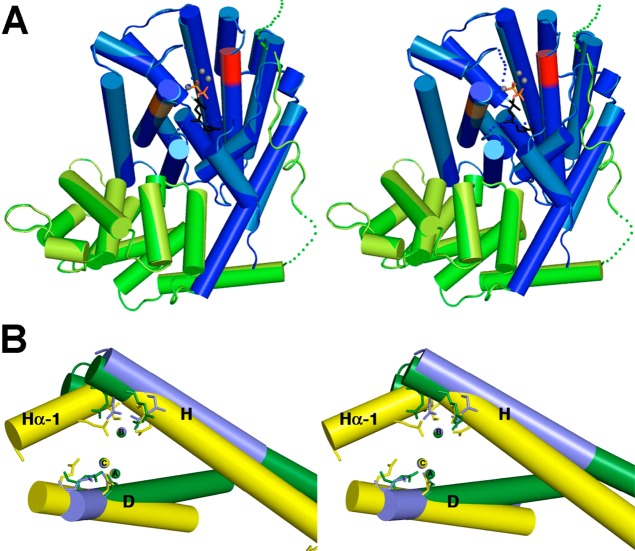
Figure 68.
(A) Stereoview showing the structure of (+)-δ-cadinene synthase complexed with 3 Mg2+ ions (gray spheres) and 2-fluorofarnesyl diphosphate (stick figure) reveals the active site in the α domain (blue) with the N-terminus of the β domain (green) partially capping the active site. Aspartate-rich metal-binding motifs on helices D and H are red and orange, respectively. (B) Stereoview showing a superposition of helices D and H of (+)-δ-cadinene synthase (blue) with those of A. terreus aristolochene synthase (yellow) and E. coli farnesyl diphosphate synthase (green). The constellation of the 3 catalytically obligatory Mg2+ ions is identical regardless of whether Mg2+B is coordinated by an aspartate-rich or NSE/DTE motif on helix H and regardless of whether the enzyme catalyzes isoprenoid coupling or cyclization reactions. Reproduced from ref (342). Copyright 2009 American Chemical Society.