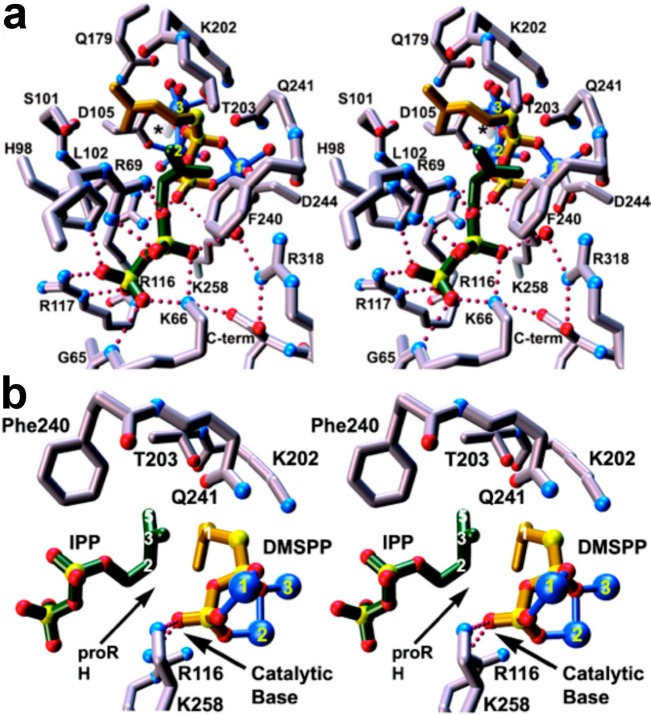
Figure 9.
(a) Stereoview of the active site of FPP synthase from E. coli complexed with IPP (C = green, O = red, and P = yellow) and the unreactive substrate analogue DMSPP (C = brown and S = yellow) reveals the binding of a full complement of 3 Mg2+ ions (blue spheres 1, 2, and 3). Metal coordination and hydrogen bond interactions are indicated by solid blue and dotted red lines, respectively. (b) Alternative orientation of the complex shown in (a) reveals that the diphosphate group of DMSPP, which ultimately becomes coproduct inorganic pyrophosphate, is suitably oriented to serve as the catalytic general base that mediates stereospecific deprotonation of the pro-R proton at C2 of IPP. Originally published in ref (65). Copyright 2004 American Society for Biochemistry & Molecular Biology.