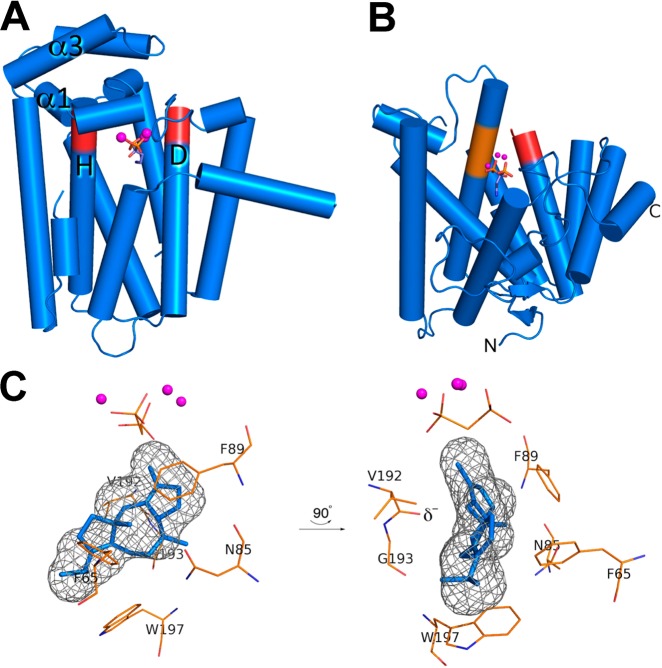
Figure 96.
(A) Crystal structure of the C-terminal GGPP synthase domain of fusicoccadiene synthase. Aspartate-rich DDXXD metal-binding motifs on helices D and H (red) coordinate to 3 Co2+ ions (magenta spheres) along with the bisphosphonate inhibitor pamidronate (stick figure), locking the active site in the fully closed conformation. (B) Crystal structure of the N-terminal cyclase domain of fusicoccadiene synthase. Aspartate-rich DDXXD and NSE metal-binding motifs on helices D and H (red and orange, respectively) coordinate to 3 Mg2+ ions (magenta spheres) along with pamidronate (stick figure), locking the active site in the fully closed conformation. (C) Molecular surface of the enclosed active site in the N-terminal cyclase domain of fusicoccadiene synthase (gray meshwork), into which the product fusicoccadiene is docked. The three-dimensional contour of the active site is very productlike and reflects the role of the active site as a template for catalysis. Reproduced from ref (95). Copyright 2016 American Chemical Society.