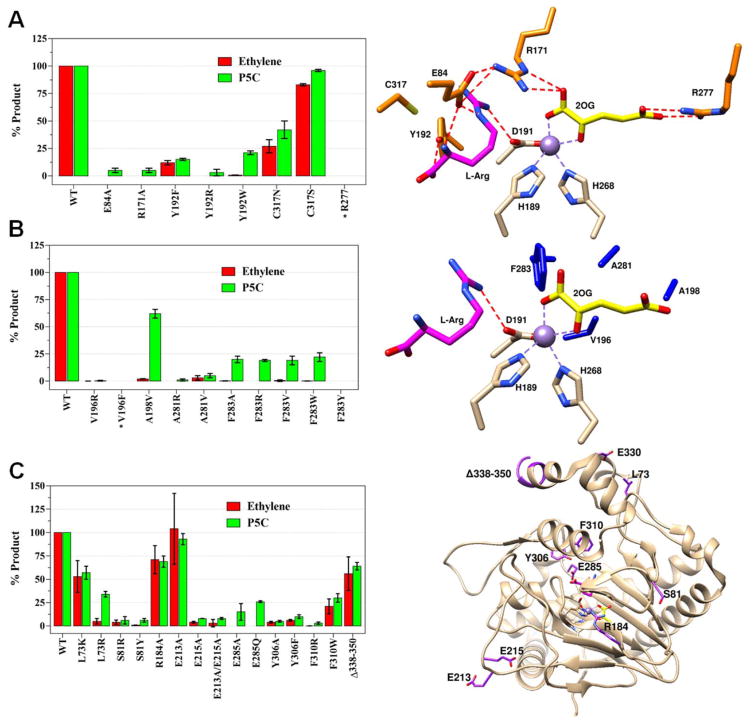
Figure 3.
Activity comparisons of WT and selected EFE variants. Ethylene and P5C production by variants of (A) selected hydrophilic residues near the active site along with their location (orange) in the EFE•Mn•2OG•Arg structure, (B) selected hydrophobic residues and their locations (dark blue), and (C) additional EFE variants and their locations (purple). Carbon atoms of metal ligands are shown in white, 2OG in yellow, and L-Arg in magenta. Mn atoms are purple spheres. Assay conditions: 2 mL reaction consisting of 0.5 mM 2OG, 0.5 mM L-Arg, 0.2 mM Fe(II), 0.4 mM L-ascorbic acid, and 1 μM EFE in 25 mM HEPES, pH 7.5, incubated at 25 °C for 80 min and terminated with HCl. WT enzyme generates ~2 ethylene per P5C, and comparisons are shown as percentage of the WT values. Error bars represent the standard errors of at least two independent enzyme preparations. Variants marked with * were found in inclusion bodies.