Figure 4.
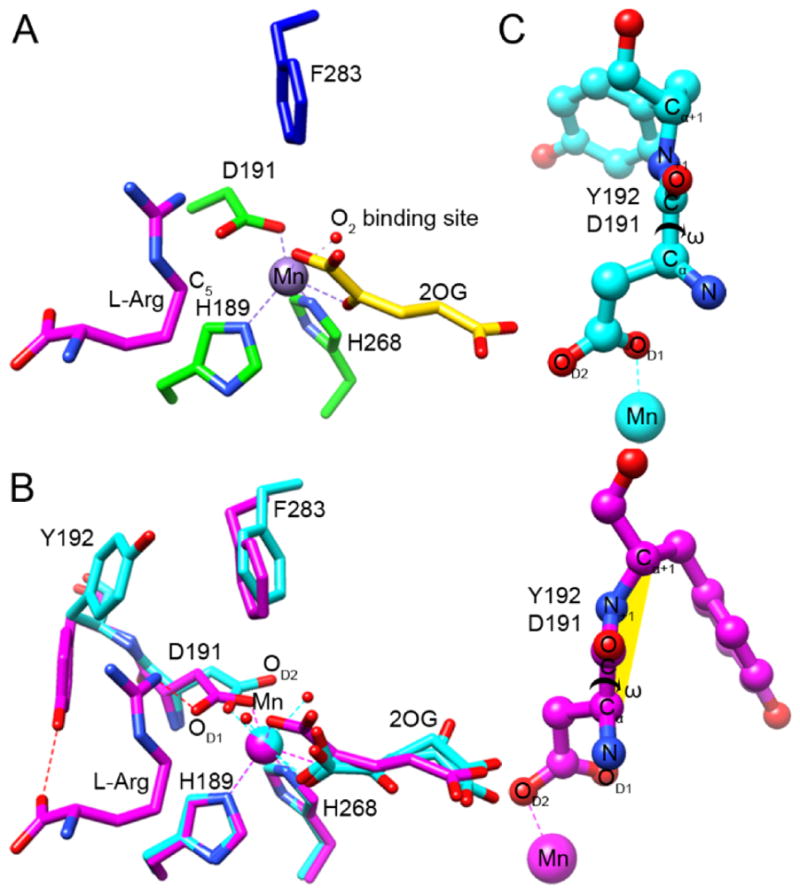
Identification of the O2 binding site and a twisted peptide bond in EFE•Mn•2OG•L-Arg. (A) The C1-carboxylate oxygen of 2OG (yellow carbons) binds approximately trans to the distal H189 and the C2-keto oxygen binds opposite D191, thus defining a dioxygen-binding site in the “off-line” configuration that points away from the C5 position of the L-Arg substrate (magenta carbons). The 2-histidine-1-carboxylate metal-binding motif is shown with green carbons, and the nearby F283 residue is shown in blue. The dioxygen-binding site is illustrated by a water molecule (red sphere) seen in our EFE•Mn•2OG•HO-L-Arg structure. (B) Conformational changes occurring upon L-Arg binding. Comparison of the active sites of EFE•Mn•2OG in cyan and EFE•Mn•2OG•L-Arg in magenta, based on an active site alignment. Metal chelation is shown by dashed lines, a key hydrogen bond between NH1 of L-Arg and OD1 of D191 is indicated by a red dashed line. Alteration of the D191 orientation leads to a switch in metal chelation from OD1 to OD2. (C) Ball and stick representations of the D191-Y192 peptide bond before and after upon L-Arg binding, showing the dihedral peptide angle ω (Cα, C, N+1, Cα+1). The twisted peptide is highlighted in yellow.
