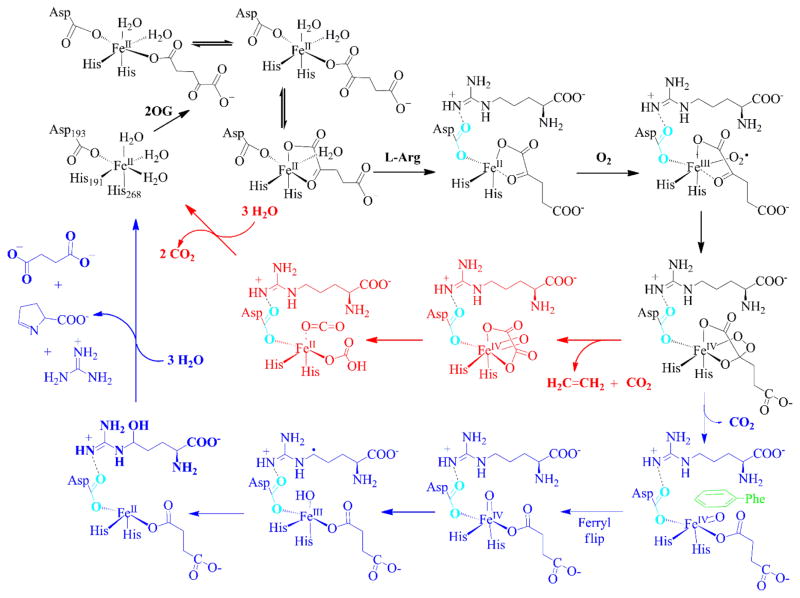
Scheme 3. Two-pathway reaction mechanism of EFE with insights provided by the enzyme structuresa.
aEFE•Fe(II) binds 2OG predominantly in two monodentate conformations, with a small amount of chelate coordination to the metal. The nearby binding of L-Arg leads to a change in orientation of D191 (shown in cyan) as it forms a hydrogen bond with this substrate and the 2OG switches to bidentate coordination. Dioxygen binds to the metallocenter opposite of L-Arg to form the Fe(III)-superoxo species that transforms to a cyclic peroxide-Fe(IV) intermediate. The mechanism diverges at this intermediate, with the predominant pathway invoking a decarboxylative fragmentation to release ethylene with oxalic acid transiently bound to the ferryl species. Subsequent reactivity of this intermediate yields CO2 and bicarbonate or two molecules of CO2 (Scheme 2) and the recycled EFE•Fe(II). The second pathway depicts oxidative decarboxylation of 2OG to yield succinate and a ferryl species pointing away from the L-Arg substrate. A ferryl flip repositions the ferryl group to point toward C5 of L-Arg, with F283 (and the repositioned D191) partly hindering this process. Hydrogen atom abstraction, hydroxyl radical rebound, elimination of guanidine from 5-hydroxy L-Arg, and ring formation complete this cycle.