Figure 3.
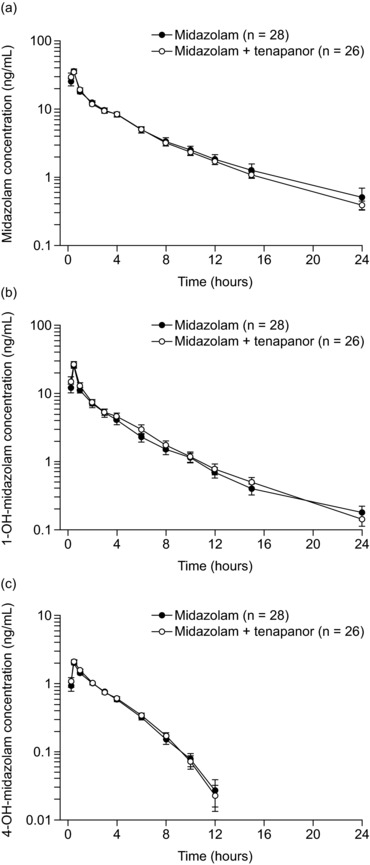
Plasma concentrations of midazolam (a) and its metabolites 1‐OH‐midazolam (b) and 4‐OH‐midazolam (c) in healthy volunteers over time following administration with and without tenapanor. Data are presented as arithmetic mean (SD). Values below the lower limit of quantification (0.1 ng/mL) were included in calculations as 0 ng/mL. Midazolam: single oral dose of midazolam 7.5 mg syrup, day 1. Midazolam + tenapanor: concurrent single oral dose of midazolam 7.5 mg and tenapanor 15‐mg tablet in the morning, day 15.
