Figure 1.
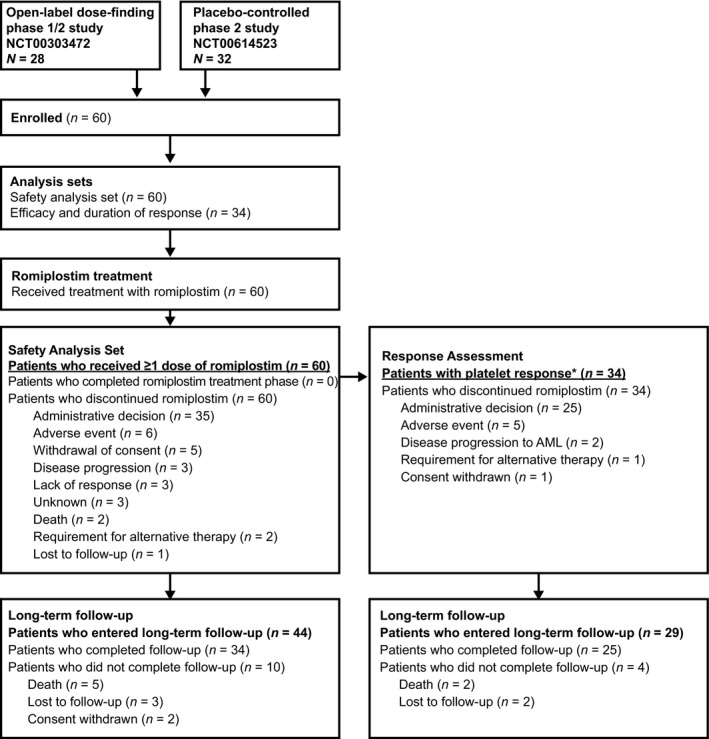
Study design and treatment schema. Patients were enrolled from one of two parent studies: (i) a 52‐week, phase 1/2 study of once‐weekly romiplostim 300–1500 μg (Kantarjian et al, 2010; Sekeres et al, 2011); (ii) a 58‐week, randomised, phase 2, placebo‐controlled study of once‐weekly romiplostim 750 μg.(Giagounidis et al, 2014) *Response per IWG 2006 criteria (Cheson et al, 2006) (Table 2). AML, acute myeloid leukaemia.
