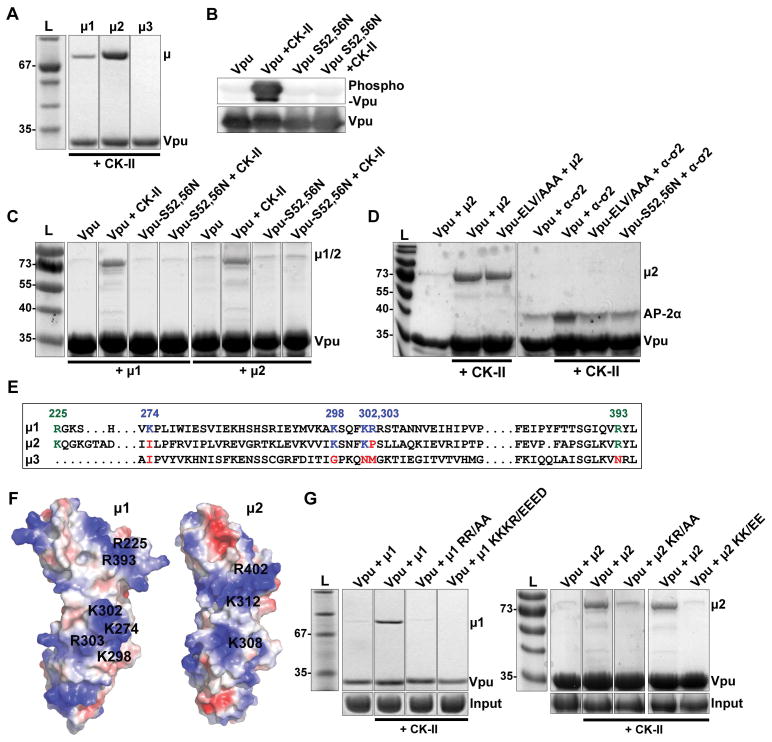
Figure 7. Vpu binds AP-1 and AP-2 subunits in a serine-phosphorylation-dependent manner.
(A) Phosphorylated Vpu binds μ1 and μ2 but not μ3 in vitro. Phosphorylated GST-tagged VpuCD recombinant protein was purified from E.Coli co-transformed to express Casein Kinase II (CK-II). The GST-VpuCD was mixed with MBP- μ1, -μ2 or -μ3 before GST-pulldown. Bound proteins were analyzed by SDS-PAGE and Coomassie staining. (B) Phosphorylation of GST-VpuCD by co-expression with CK-II was confirmed by western blot using an antibody which specifically detects Vpu phosphorylated at serines 52 and 56. (C) Phosphorylation of Serines 52 and 56 is required for μ1 and μ2 binding. GST-pulldown of μ1 and μ2 was evaluated using Vpu, Vpu-S52/56N or Vpu and Vpu-S52/56N produced in the presence of Casein Kinase II (CK-II). (D) Phosphorylation of serines 52 and 56 increases Vpu’s binding affinity for both the AP-2 α-σ2 hemicomplex and μ2. GST-pulldown of AP-2 μ2 and α-σ2 was evaluated using either Vpu, or phosphorylated Vpu or the indicated Vpu mutants produced in the presence of Casein Kinase II (CK-II). (E) Analogous basic residues present in μ1 and μ2, but not μ3, as shown in a structure based-sequence alignment. (F) These basic residues occupy similar regions in μ1 and μ2; positively-charged residues are shown in blue, negative in red. (G) Basic residues in μ1 and μ2 are required for binding phosphorylated Vpu. GST-pulldown was performed between phosphorylated Vpu and μ1 or μ2 containing substitutions within the two indicated sets of basic residues; R225, R393/AA and K274, K298, K302, R303/EEED in μ1, and equivalent residues K227, R402/AA and K308, K312/EE in μ2.