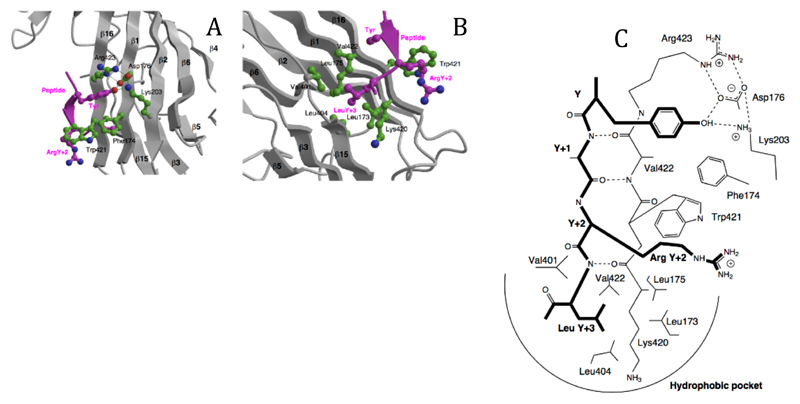
Figure 3. The peptide binding site.
A The binding of the tyrosine residue of the internalisation signal peptide is in a hydrophobic pocket created by Phe174, Trp421 and Arg423, with a hydrogen-bonding network between the tyrosine OH and Asp176, Lys203 and Arg423. The structure shown is that of the DYQRLN TGN38 peptide. B The binding pocket for the bulky hydrophobic residue at Y+3 (Leucine in both peptides) is lined with aliphatic sidechains of Leu173, Leu175, Val401, Leu404, Val422 and the aliphatic portion of Lys420. ArgY+2 of the TGN38 peptide is packed against Trp421. C Schematic representation of the interactions between the internalisation signal of TGN38 and μ2, showing both side chain contacts and the short stretch of β-sheet formed between the peptide and β-strand 16. The peptide is shown with bold lines.