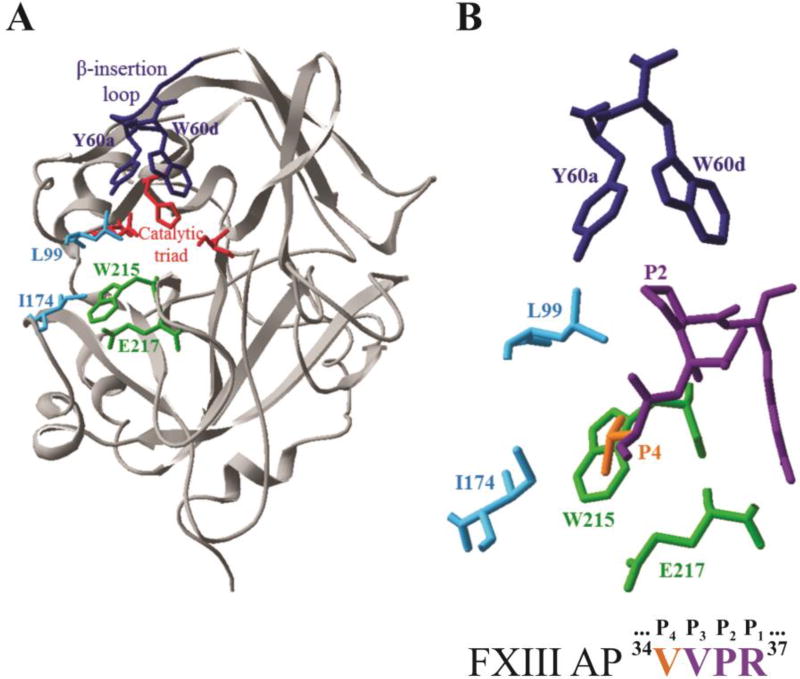
Figure 1.
X-ray crystal structure of thrombin highlighting key residues that surround the bound structure of FXIII AP (34–37). A) Ribbon diagram of thrombin (gray) showing selected residues as colored sticks (PDB ID: 1DE7). Residues include the catalytic triad (red), Y60a and W60d (blue), L99 and I174 (cyan), W215 and E217 (green). B) The enzyme-bound FXIII AP segment (34V orange, 35VPR37 purple) surrounded by thrombin residues that were mutated to alanines for the kinetic studies (PDB ID: 1DE7).