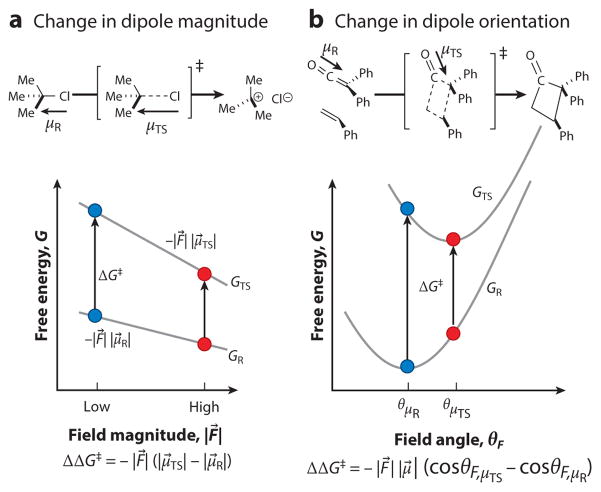
Figure 2.
Two limiting cases of electric field catalysis. Electric field catalysis lowers a reaction’s activation barrier by ΔΔG‡ = −((F⃗TS · μ⃗TS) − (F⃗R · μ⃗R)). This expression simplifies in two limiting scenarios, where blue dots represent a reference case and red dots represent catalysis. (a) If the transition state’s (TS) and reactant’s (R) dipole moments are oriented in the same direction but the transition state’s dipole is greater (such as in the rate-determining heterolysis step of an SN1 reaction), larger electric fields (such as provided by a polar solvent) will catalyze the reaction. (b) If the transition state’s and reactant’s dipole moments have the same magnitude but have different orientations (such as is approximately true in the cycloaddition of a ketene), the magnitude of the electric field will not have a catalytic effect. Electric field catalysis can be achieved only by “correctly” orienting the field, that is, aligning it with the transition state’s dipole orientation instead of the reactant’s dipole orientation.