Fig. 4. MxB intermolecular assembly interfaces and their role in the MxB assembly and HIV-1 inhibition.
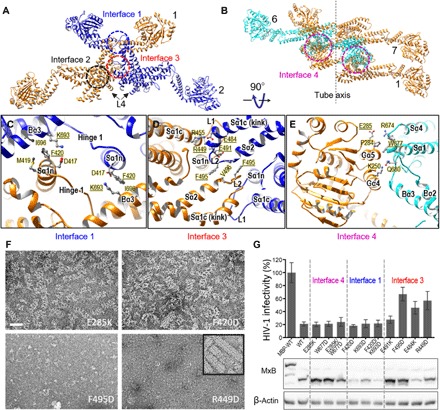
(A) Intermolecular interfaces in an MxB oligomer: the canonical MxB dimer interface, interface 2 (black circle), and the lateral interfaces that link adjacent MxB dimers, interfaces 1 (blue circle) and 3 (red circle). The same color scheme is used as in Fig. 3F. (B) Interface 4 (magenta circles), the vertical interface between adjacent rungs. MxB dimer 7 starts the next rung in the helix, colored the same as dimer 1. (C to E) Expanded views of the intermolecular interfaces: interface 1 (C), interface 3 (D), and interface 4 (E). Specific residues at the interfaces are labeled, along with the secondary structures. Underlined amino acids were subjected to mutational analysis. (F) Effects of interface mutations on the MxB assembly: E285K (interface 4), F420D (interface 1), F495D, and R449D (interface 3). Negatively stained images of purified interface mutant proteins under helical assembly conditions are shown. Inset, wild-type MxB. Scale bar, 50 nm. (G) Effects of interface mutations on MxB anti–HIV-1 activity (top) (mean ± SD for minimally n = 3 independent experiments) and Western blot analysis of MxB protein expression (bottom). MBP-tagged wild-type (WT) MxB, which does not inhibit HIV-1, was used as a control.
