Abstract
Objective
To describe sedation management in children supported on extracorporeal membrane oxygenation (ECMO) for acute respiratory failure.
Design
Secondary analysis of prospectively collected data from a multicenter randomized trial of sedation (RESTORE).
Setting
21 U.S. Pediatric Intensive Care Units.
Patients
1255 children, 2 weeks to 17 years old, with moderate/severe PARDS.
Interventions
Sedation managed per usual care or RESTORE protocol.
Measurements and Main Results
Sixty-one (5%) RESTORE patients with moderate/severe PARDS were supported on ECMO, including 29 managed per RESTORE protocol. Most ECMO patients received neuromuscular blockade (46%) or were heavily sedated with SBS scores −3/−2 (34%) by ECMO day 3. Median opioid and benzodiazepine doses on the day of cannulation, 0.15 mg/kg/hr (3.7 mg/kg/day) and 0.11 mg/kg/hr (2.8 mg/kg/day), increased by 36% and 58%, respectively, by ECMO day 3. In the 41 patients successfully decannulated prior to study discharge, patients were receiving 0.40 mg/kg/hr opioids (9.7 mg/kg/day) and 0.39 mg/kg/hr benzodiazepines (9.4 mg/kg/day) at decannulation, an increase from cannulation of 108% and 192%, respectively (both p<0.001). ECMO patients experienced more clinically significant iatrogenic withdrawal than moderate/severe PARDS patients managed without ECMO support (p<0.001). Compared to ECMO patients managed per RESTORE protocol, usual care ECMO patients received more opioids during the study period (mean cumulative dose of 183.0 vs 89.8 mg/kg; p=0.02), over 6.5 greater exposure days (p=0.002) with no differences in wakefulness or agitation.
Conclusions
In children, the initiation of ECMO support is associated with deep sedation, substantial sedative exposure, and increased incidence of iatrogenic withdrawal syndrome. A standardized, goal-directed, nurse-driven sedation protocol may help mitigate these effects.
Keywords: analgesia, agitation, iatrogenic withdrawal syndrome, State Behavioral Scale, Withdrawal Assessment Tool – Version 1, pediatric intensive care
Introduction
Sedation management is a ubiquitous and important aspect of pediatric critical care. The goals of sedation include maintenance of comfort, avoidance of agitation, and patient safety(1). Therapeutic sedation risks hemodynamic and respiratory depression in the short-term and tolerance, physical dependence, iatrogenic withdrawal syndrome (IWS), and potential for neurotoxicity in the long-term(2)(3). Recent focus has centered on identifying novel approaches that provide adequate sedation while minimizing these untoward effects(4).
Sedation goals may be more difficult to achieve in pediatric patients undergoing extracorporeal membrane oxygenation (ECMO). Previous studies have shown that ECMO circuits alter the pharmacokinetics and pharmacodynamics of sedative medications(5–9), although these effects with contemporary ECMO circuits are not well understood. These alterations are related to medication absorption by components of the ECMO circuit, where up to 40% of lorazepam and 50% of morphine doses can be extracted(10, 11). In addition, ECMO-related physiologic and metabolic alterations raise concern for variations in sedative requirements in ECMO patients(12). Escalating sedative requirements, as well as development of IWS, have been well-described in the neonatal ECMO population.(13, 14).
Although ECMO circuit-related pharmacokinetic alterations in opioid and benzodiazepine concentrations are well-known, little knowledge exists on the clinical effects of these issues outside the neonatal population, and an optimal approach to patient sedation on ECMO has not been achieved(15). The purpose of this paper is to describe sedation management in pediatric patients supported on ECMO for severe respiratory failure and to contrast sedation management using a nurse-implemented goal-directed sedation strategy to usual care.
Materials and Methods
We performed a secondary analysis of prospectively collected data from the RESTORE (Randomized Evaluation of Sedation Titration for Respiratory Failure) clinical trial. RESTORE was a multicenter cluster randomized trial that compared a nurse-implemented goal-directed sedation strategy to usual care in children 2 weeks to 17 years of age with acute respiratory failure secondary to airways or parenchymal lung disease. The study protocol and findings have been described elsewhere(4). Essential elements included daily team discussion of the patient’s trajectory of illness (acute, titration, or weaning phase); prescribing a State Behavioral Scale (SBS)(16) target per phase of illness; arousal assessments if the patient was over sedated in the titration/weaning phases; daily extubation readiness testing; titrating sedatives at least every 8 hours; and either discontinuing or weaning sedatives per target Withdrawal Assessment Tool-Version 1(WAT-1)(17) based on length of exposure. This study was reviewed and approved by the institutional review board of each participating site.
Assessments of pain(18–20), sedation(16), and IWS(17) were standardized in all participating Pediatric Intensive Care Units (PICUs). Bedside care teams at intervention PICUs assigned a daily target sedation score per patient’s phase of illness, and nurses used an algorithm to titrate sedatives to achieve the prescribed goal. Primary sedatives were morphine and midazolam. Per algorithm, patients supported on ECMO were considered to be in their acute phase of illness where the sedation goal is to maintain the status quo with an SBS of −1 (responsive to gentle touch or voice) or lower if deemed appropriate by the care team.
The RESTORE database includes demographic information, medical history data, baseline Pediatric Cerebral Performance Category (PCPC) and Pediatric Overall Performance Category (POPC)(21), Pediatric Risk of Mortality (PRISM) III-12 scores(22), daily organ function scores, comfort assessments, and sedative dosing data from endotracheal intubation to study discharge (72 hours after the last opioid dose, day 28, or hospital discharge). Sedation profiles include total sedative exposure, use of neuromuscular blockade, sedation-related adverse events, and measures of wakefulness, pain, and agitation. ECMO data include mode of cannulation, equipment type, and center volume. Centers with >10 ECMO cases in a calendar year were defined as high volume centers for that year.
Statistical Analysis
Baseline patient characteristics were compared between ECMO patients and those not supported on ECMO. To facilitate appropriate comparisons, we excluded all data from sites not contributing at least one ECMO study patient and from all lower acuity patients unlikely to be supported on ECMO; specifically, patients with an oxygenation index <8.0 or oxygenation saturation index <7.5(23) on the first two days after intubation.
For patients supported on ECMO, sedatives, sedation-related adverse events, and SBS scores are presented by day preceding, during, and subsequent to cannulation and decannulation (±3 days), and sedative dose changes over time were evaluated. Clinical variables and outcomes surrounding cannulation and decannulation were considered separately to more clearly describe these clinically independent events during the course of ECMO. For cannulation, we included data from all ECMO patients. For decannulation, we only included data from ECMO patients successfully decannulated during the study period. In this subset of patients, we compared sedative dosing during ECMO and neuromuscular blockade use at decannulation by mode of ECMO support (veno-venous [VV] vs veno-arterial [VA]), circuit configuration (polymethylpentene vs polypropylene oxygenator, roller vs centrifugal pump), age (<2 vs ≥2 years), and high vs low volume centers. We also compared sedative dosing during ECMO for patients who were co-managed with NMB for at least half of their ECMO course vs those who were not and those requiring renal replacement therapy (RRT) vs no RRT.
To assess the clinical impact of the RESTORE sedation protocol, sedative profiles were compared between ECMO patients in the usual care and intervention groups. We compared sedation requirements between ECMO and non-ECMO intervention group patients during their acute phase of illness. In addition, the frequency of IWS was compared between the ECMO and non-ECMO groups, and between ECMO patients in the usual care and intervention groups.
All group comparisons were performed using linear, logistic, multinomial logistic, cumulative logit, and proportional hazards regression accounting for PICU as a cluster variable using generalized estimating equations for continuous, binary, nominal, ordinal, and time-to-event variables, respectively. Continuous variables except percentage of study days variables were log-transformed. The statistical significance of sedative dose changes over time were evaluated using intercept-only linear regression. All data analyses were performed using SAS (Version 9.4, SAS Institute, Cary, NC).
Results
Of 2,449 RESTORE patients from 31 PICUs, we excluded 482 patients from 10 PICUs that did not enroll an ECMO patient as well as 712 patients with at-risk/mild PARDS from the remaining 21 PICUs (9 usual care, 12 intervention). The 21 PICUs supported a median of 25 patients annually on ECMO (range, 2–77). Most centers (n=18) supported more than 10 patients per year on ECMO during the course of the study. Sixty-one of the remaining 1,255 study patients (5%) were supported with ECMO, specifically VV ECMO (n=38, 62%) or VA ECMO (n=23, 38%). ECMO equipment varied, with polymethylpentene oxygenators used for 34 patients (56%), polypropylene for 23 (38%), and silicone for 4 (7%). Centrifugal pumps were more commonly used (n=35, 57%) than roller pumps.
Table 1 describes the baseline characteristics of the 61 patients supported on ECMO compared to the 1,194 patients with moderate/severe PARDS not supported on ECMO. ECMO patients had more severe PARDS and exhibited more organ dysfunction on days 0 to 1. Over half of the 61 ECMO patients were cannulated by day 3 (median; interquartile range [IQR], 1–6) post-intubation. Patients remained on ECMO support for a median of 9 study days prior to study discharge (IQR, 6–14). Twenty-eight ECMO patients (46%) were successfully decannulated and extubated by the end of the study period, with a median length of intubation post-decannulation of 4 days (IQR, 3–8.5). In addition, 13 (21%) were decannulated but remained intubated and 20 (33%) were not decannulated prior to study discharge. Of the 20 patients not decannulated, 10 died while on ECMO support, 8 were still on ECMO on day 28, and 2 were still on ECMO upon transfer to a non-participating PICU.
Table 1.
Patient Characteristics According to Group
| Characteristics | ECMO (n = 61) | No ECMO (n = 1194) | pa |
|---|---|---|---|
| Age at PICU admission, median (IQR), y | 4.2 (0.8–12.0) | 2.6 (0.5–9.3) | 0.04 |
| Female sex, n (%) | 39 (64) | 563 (47) | <0.001 |
| Non-Hispanic white, n/total (%) | 30/57 (53) | 620/1183 (52) | 0.80 |
| Cognitive impairment (baseline PCPC score >1), n (%) | 6 (10) | 301 (25) | 0.002 |
| Functional impairment (baseline POPC score >1), n (%) | 9 (15) | 363 (30) | 0.006 |
| PRISM III-12 score, median (IQR) | 12 (4–21) | 8 (3–14) | 0.09 |
| Percent risk of mortality based on PRISM III-12 score, median (IQR) | 13.1 (1.7–42.6) | 4.6 (1.3–15.0) | 0.02 |
| Primary diagnosis, n (%) | 0.09 | ||
| Pneumonia | 28 (46) | 411 (34) | |
| Bronchiolitis | 8 (13) | 292 (24) | |
| Acute respiratory failure related to sepsis | 14 (23) | 197 (17) | |
| Asthma or reactive airway disease | 6 (10) | 87 (7) | |
| Aspiration pneumonia | 1 (2) | 71 (6) | |
| Otherb | 4 (7) | 136 (11) | |
| Past medical history, n (%) | |||
| Prematurity (<36 wk postmenstrual age) | 8 (13) | 188 (16) | 0.58 |
| Asthma (prescribed bronchodilators or steroids) | 7 (11) | 180 (15) | 0.43 |
| Seizure disorder (prescribed anticonvulsants) | 3 (5) | 108 (9) | 0.22 |
| Cancer (current or previous diagnosis) | 8 (13) | 123 (10) | 0.47 |
| Known chromosomal abnormality | 2 (3) | 60 (5) | 0.48 |
| Intervention group, n (%) | 29 (48) | 582 (49) | 0.92 |
| PARDS based on worst OI or OSI on Days 0 to 1, n (%)c | <0.001 | ||
| Moderate (OI 8.0–15.9 or OSI 7.5–12.2) | 4 (7) | 576 (48) | |
| Severe (OI ≥16.0 or OSI ≥12.3) | 57 (93) | 618 (52) | |
| Organ dysfunctions on Days 0 to 1, median (IQR)d | 3 (2–4) | 2 (1–3) | <0.001 |
| Neuromuscular blockade for the entire duration of Days 0 to 2, n (%) | 15 (25) | 115 (10) | 0.009 |
ECMO = extracorporeal membrane oxygenation, IQR = interquartile range, OI = oxygenation index, OSI = oxygen saturation index, PARDS = pediatric acute respiratory distress syndrome, PCPC = Pediatric Cerebral Performance Category, PICU = pediatric intensive care unit, POPC = Pediatric Overall Performance Category, PRISM III-12 = Pediatric Risk of Mortality III score from first 12 hours in the PICU.
P values for the comparison between groups were calculated using linear, logistic, multinomial logistic, and cumulative logit regression for log-transformed continuous, binary, nominal, and ordinal variables, respectively. All regression analyses except for primary diagnosis accounted for PICU as a cluster variable using generalized estimating equations.
Other primary diagnoses include pulmonary edema, thoracic trauma, pulmonary hemorrhage, laryngotracheobronchitis, acute respiratory failure after bone marrow transplantation, acute chest syndrome/sickle cell disease, pertussis, pneumothorax (nontrauma), acute exacerbation lung disease (cystic fibrosis or bronchopulmonary dysplasia), acute respiratory failure related to multiple blood transfusions, pulmonary hypertension (not primary), and pulmonary embolus.
Oxygenation index was calculated as ([FIO2 × mean airway pressure]/PaO2 × 100). When an arterial blood gas measurement was not available, SpO2 was used to estimate PaO2 in order to calculate OSI ([FIO2 × mean airway pressure]/SpO2 × 100). Lower scores reflect better oxygenation.
Number of organ dysfunctions ranges from 1 to 6. All patients had respiratory dysfunction. Cardiovascular dysfunction based on vasoactive medication use (single or multiple). Neurologic dysfunction based on worst level of consciousness (stupor or coma) or pupillary response (one or both pupils non-reactive). Hematologic dysfunction based on platelet threshold (<80 K/μL). Renal dysfunction based on age-specific creatinine thresholds. Hepatic dysfunction based on age- and gender-specific ALT thresholds or total bilirubin thresholds.
Table 2 shows daily sedation profiles around cannulation of all 61 ECMO patients. On the day of cannulation, median doses of opioid and benzodiazepines received were 0.15 mg/kg/hr (3.7 mg/kg/day) and 0.11 mg/kg/hr (2.8 mg/kg/day), respectively. With little change in sedative dosing in the three days prior to cannulation, significant increases in opioid and benzodiazepine doses were identified on each of the three days post-cannulation (all p<0.05). By the third day post-cannulation, median daily opioid and benzodiazepine dose increased by 36% and 58%, respectively, from the day of cannulation for the 58 patients still on ECMO support. On this day, almost half of the patients were receiving neuromuscular blockade (NMB), approximately one third had modal SBS scores of −3/−2, and the remaining were more awake.
Table 2.
Sedation Profiles of ECMO Patients By Day around Cannulation
| Variables | 3 days earlier (n = 31) | 2 days earlier (n = 35) | 1 day earlier (n = 52) | Day of cannulation (n = 61) | 1 day later (n = 61) | 2 days later (n = 61) | 3 days later (n = 58) |
|---|---|---|---|---|---|---|---|
| Sedatives administered | |||||||
| Opioid dose, median (IQR), mg/kga | 3.2 (1.5–6.5) | 3.5 (1.8–6.8) | 3.7 (1.4–6.2) | 3.7 (2.4–5.6) | 4.9 (2.8–6.9) | 4.6 (2.8–8.1) | 5.2 (3.4–9.8) |
| Number of opioid bolus doses, median (IQR) | 3 (1–6) | 4 (1–6) | 4 (2–6.5) | 4 (2–6) | 4 (1–7) | 4 (2–7) | 6 (1–9) |
| Benzodiazepine dose, median (IQR), mg/kgb | 3.0 (1.3–7.3) | 3.4 (2.0–7.4) | 2.9 (0.9–6.3) | 2.8 (1.2–6.5) | 3.8 (2.2–7.8) | 3.7 (2.2–9.6) | 5.2 (3.4–11.2) |
| Number of benzodiazepine bolus doses, median (IQR) | 3 (1–5) | 3 (1–6) | 3 (1–7) | 2 (1–5) | 3 (1–6) | 3 (1–8) | 4 (1–8) |
| Secondary sedatives, n (%) | |||||||
| Dexmedetomidine | 6 (19) | 7 (20) | 8 (15) | 8 (13) | 11 (18) | 13 (21) | 10 (17) |
| Propofol | 0 | 0 | 0 | 2 (3) | 0 | 1 (2) | 0 |
| Barbiturates | 2 (6) | 1 (3) | 3 (6) | 4 (7) | 5 (8) | 8 (13) | 7 (12) |
| Ketamine | 3 (10) | 2 (6) | 6 (12) | 10 (16) | 3 (5) | 5 (8) | 6 (10) |
| Clonidine | 2 (6) | 3 (9) | 3 (6) | 4 (7) | 3 (5) | 3 (5) | 4 (7) |
| Chloral hydrate | 0 | 0 | 0 | 0 | 1 (2) | 1 (2) | 1 (2) |
| Number of different sedative classes received, median (IQR)c | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) |
| Sedation-related adverse eventsd | |||||||
| Inadequate pain management, n (%) | 0 | 0 | 2 (4) | 0 | 0 | 3 (5) | 1 (2) |
| Inadequate sedation management, n (%) | 0 | 1 (3) | 3 (6) | 0 | 2 (3) | 3 (5) | 2 (3) |
| Sedation scorese | (n = 27) | (n = 35) | (n = 47) | (n = 59) | (n = 56) | (n = 58) | (n = 56) |
| Modal SBS score, n (%) | |||||||
| +1/+2 | 0 | 0 | 1 (2) | 0 | 0 | 2 (3) | 2 (4) |
| −1/0 | 6 (22) | 5 (14) | 4 (9) | 4 (7) | 6 (11) | 6 (10) | 9 (16) |
| −3/−2 | 7 (26) | 12 (34) | 9 (19) | 15 (25) | 20 (36) | 23 (40) | 19 (34) |
| Neuromuscular blockade entire day | 14 (52) | 18 (51) | 33 (70) | 40 (68) | 30 (54) | 27 (47) | 26 (46) |
| Highest SBS score, n (%) | |||||||
| +1/+2 | 3 (11) | 3 (9) | 4 (9) | 4 (7) | 7 (13) | 7 (12) | 10 (18) |
| −1/0 | 8 (30) | 7 (20) | 2 (4) | 3 (5) | 9 (16) | 13 (22) | 10 (18) |
| −3/−2 | 2 (7) | 7 (20) | 8 (17) | 12 (20) | 10 (18) | 11 (19) | 10 (18) |
| Neuromuscular blockade entire day | 14 (52) | 18 (51) | 33 (70) | 40 (68) | 30 (54) | 27 (47) | 26 (46) |
ECMO = extracorporeal membrane oxygenation, IQR = interquartile range, SBS = State Behavioral Scale.
Opioid doses were calculated as morphine equivalents in mg/kg, including morphine (1), fentanyl (0.015), methadone (0.3), enteral codeine (20), hydromorphone (0.15), enteral oxycodone (3), and remifentanil (0.015).
Benzodiazepine doses were calculated as midazolam equivalents in mg/kg, including midazolam (1), clonazepam (0.2), lorazepam (0.3), and diazepam (2).
Different sedatives classes include opioids, benzodiazepines, α2-adrenergic agonists, propofol, barbiturates, ketamine, and chloral hydrate.
Inadequate pain management: pain score >4 [or pain assumed present if receiving neuromuscular blockade] for 2 consecutive hours; inadequate sedation management: SBS score >0 [or agitation assumed present if receiving neuromuscular blockade] for 2 consecutive hours.
The SBS scores range from −3 (unresponsive) to +2 (agitated).
Table 3 shows daily sedation profiles around decannulation of the 41 ECMO patients successfully decannulated by the end of the study period. On the day of decannulation, patients were receiving 0.40 mg/kg/hr opioids (9.7 mg/kg/day) and 0.39 mg/kg/hr benzodiazepines (9.4 mg/kg/day). Opioid and benzodiazepine doses increased significantly from cannulation to decannulation day (p<0.001 for each). Median daily opioid and benzodiazepine doses increased 108% and 192%, respectively, over this time period for the 41 patients successfully decannulated prior to study discharge. Contrary to the time after cannulation, opioid and benzodiazepine doses significantly decreased for the three days post-decannulation (all p<0.05). For the 40 patients still on study, by the third day post-decannulation, median daily opioid and benzodiazepine doses decreased by 35% and 24%, respectively, and modal SBS scores shifted to an awake state.
Table 3.
Sedation Profiles of ECMO Patients By Day around Decannulationa
| Variables | 3 days earlier (n = 39) | 2 days earlier (n = 41) | 1 day earlier (n = 41) | Day of decannulation (n = 41) | 1 day later (n = 41) | 2 days later (n = 41) | 3 days later (n = 40) |
|---|---|---|---|---|---|---|---|
| Sedatives administered | |||||||
| Opioid dose, median (IQR), mg/kgb | 7.3 (3.7–11.2) | 7.7 (4.3–11.5) | 7.8 (4.9–12.5) | 9.7 (5.2–14.5) | 8.1 (4.8–13.2) | 7.1 (4.4–11.3) | 5.1 (3.0–12.3) |
| Number of opioid bolus doses, median (IQR) | 5 (2–7) | 5 (2–8) | 5 (3–8) | 5 (3–8) | 3 (1–6) | 4 (1–7) | 1.5 (0–5) |
| Benzodiazepine dose, median (IQR), mg/kgc | 6.2 (3.6–12.6) | 7.3 (4.3–11.7) | 7.7 (5.1–12.6) | 9.4 (6.2–13.0) | 8.7 (4.8–12.6) | 7.5 (4.8–13.3) | 6.5 (3.6–12.5) |
| Number of benzodiazepine bolus doses, median (IQR) | 4 (2–7) | 4 (1–9) | 5 (2–7) | 5 (1–8) | 4 (1–6) | 3 (1–6) | 1.5 (0–5.5) |
| Secondary sedatives, n (%) | |||||||
| Dexmedetomidine | 10 (26) | 12 (29) | 11 (27) | 11 (27) | 14 (34) | 13 (32) | 14 (35) |
| Propofol | 0 | 1 (2) | 2 (5) | 2 (5) | 0 | 0 | 1 (3) |
| Barbiturates | 7 (18) | 10 (24) | 12 (29) | 13 (32) | 12 (29) | 9 (22) | 8 (20) |
| Ketamine | 5 (13) | 4 (10) | 5 (12) | 7 (17) | 5 (12) | 2 (5) | 3 (8) |
| Clonidine | 3 (8) | 4 (10) | 4 (10) | 4 (10) | 4 (10) | 5 (12) | 5 (13) |
| Methadone | 1 (3) | 1 (2) | 1 (2) | 1 (2) | 6 (15) | 10 (24) | 15 (38) |
| Chloral hydrate | 1 (3) | 1 (2) | 1 (2) | 1 (2) | 3 (7) | 3 (7) | 2 (5) |
| Number of different sedative classes received, median (IQR)d | 2 (2–3) | 2 (2–3) | 3 (2–3) | 3 (2–3) | 3 (2–3) | 3 (2–3) | 2.5 (2–3) |
| Sedation-related adverse eventse | |||||||
| Inadequate pain management, n (%) | 3 (8) | 1 (2) | 2 (5) | 0 | 2 (5) | 3 (7) | 2 (5) |
| Inadequate sedation management, n (%) | 2 (5) | 2 (5) | 2 (5) | 2 (5) | 4 (10) | 6 (15) | 3 (8) |
| Clinically significant iatrogenic withdrawal, n (%) | 0 | 0 | 0 | 0 | 0 | 1 (2) | 2 (5) |
| Sedation scoresf | (n = 38) | (n = 39) | (n = 38) | (n = 40) | (n = 40) | (n = 37) | (n = 39) |
| Modal SBS score, n (%) | |||||||
| +1/+2 | 0 | 0 | 0 | 0 | 3 (8) | 1 (3) | 3 (8) |
| −1/0 | 8 (21) | 13 (33) | 10 (26) | 12 (30) | 22 (55) | 27 (73) | 24 (62) |
| −3/−2 | 11 (29) | 6 (15) | 8 (21) | 9 (23) | 6 (15) | 3 (8) | 3 (8) |
| Neuromuscular blockade entire day | 19 (50) | 20 (51) | 20 (53) | 19 (48) | 9 (23) | 6 (16) | 3 (8) |
| No longer intubated | 0 | 0 | 0 | 0 | 0 | 0 | 6 (15) |
| Lowest SBS score, n (%) | |||||||
| +1/+2 | 0 | 0 | 0 | 0 | 1 (3) | 0 | 0 |
| −1/0 | 3 (8) | 6 (15) | 5 (13) | 8 (20) | 14 (35) | 22 (59) | 23 (59) |
| −3/−2 | 16 (42) | 13 (33) | 13 (34) | 13 (33) | 16 (40) | 9 (24) | 7 (18) |
| Neuromuscular blockade entire day | 19 (50) | 20 (51) | 20 (53) | 19 (48) | 9 (23) | 6 (16) | 3 (8) |
| No longer intubated | 0 | 0 | 0 | 0 | 0 | 0 | 6 (15) |
| Highest SBS score, n (%) | |||||||
| +1/+2 | 6 (16) | 9 (23) | 7 (18) | 10 (25) | 14 (35) | 20 (54) | 22 (56) |
| −1/0 | 9 (24) | 9 (23) | 10 (26) | 8 (20) | 15 (38) | 11 (30) | 7 (18) |
| −3/−2 | 4 (11) | 1 (3) | 1 (3) | 3 (8) | 2 (5) | 0 | 1 (3) |
| Neuromuscular blockade entire day | 19 (50) | 20 (51) | 20 (53) | 19 (48) | 9 (23) | 6 (16) | 3 (8) |
| No longer intubated | 0 | 0 | 0 | 0 | 0 | 0 | 6 (15) |
ECMO = extracorporeal membrane oxygenation, IQR = interquartile range, SBS = State Behavioral Scale.
Excluded 20 patients who were not decannulated prior to study discharge (10 patients died on ECMO support, 8 patients still on ECMO on Day 28, and 2 patients still on ECMO on day of transfer to non-participating pediatric intensive care unit).
Opioid doses were calculated as morphine equivalents in mg/kg, including morphine (1), fentanyl (0.015), methadone (0.3), enteral codeine (20), hydromorphone (0.15), enteral oxycodone (3), and remifentanil (0.015).
Benzodiazepine doses were calculated as midazolam equivalents in mg/kg, including midazolam (1), clonazepam (0.2), lorazepam (0.3), and diazepam (2).
Different sedatives classes include opioids, benzodiazepines, α2-adrenergic agonists, propofol, barbiturates, ketamine, and chloral hydrate.
Inadequate pain management: pain score >4 [or pain assumed present if receiving neuromuscular blockade] for 2 consecutive hours; inadequate sedation management: SBS score >0 [or agitation assumed present if receiving neuromuscular blockade] for 2 consecutive hours; clinically significant iatrogenic withdrawal in patients weaning from 5 or more days of opioids: rescue therapy to manage an increase in WAT-1 symptoms.
The SBS scores range from −3 (unresponsive) to +2 (agitated).
Restricting to the 41 patients successfully decannulated prior to study discharge, there were no differences in sedation dosing during ECMO or the percentage of patients receiving NMB on the day of decannulation by ECMO mode, center volume, or age group. Those supported on polymethylpentene vs polypropylene oxygenators and/or roller vs centrifugal pumps received higher mean daily benzodiazepine doses during ECMO (median 0.33 vs 0.19 mg/kg/hr, p<0.001; 0.34 vs 0.21 mg/kg/hr, p=0.001, respectively), but similar opioid doses and NMB use at decannulation. There were no differences in sedation dosing during ECMO between patients co-managed with NMB and those who were not. RRT patients (N=16) had more study days on ECMO (median 11.5 vs 6 days, p<0.001) and were exposed to more opioids compared to non-RRT patients; median mean daily dose 0.38 vs 0.24 mg/kg/hr (p=0.050) and median cumulative dose 88.6 vs 36.6 mg/kg (p=0.004).
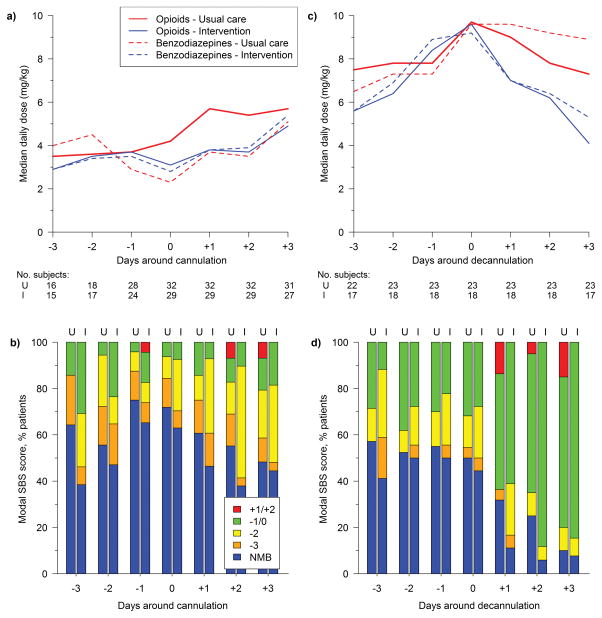
We compared sedation profiles of ECMO patients by RESTORE treatment group (32 usual care, 29 intervention). There were no treatment group differences in either opioid or benzodiazepine daily doses during three days pre-cannulation through three days post-cannulation, (Figure 1). Restricting to the 41 patients successfully decannulated prior to study discharge (23 usual care, 18 intervention), usual care patients received more opioids during the entire study period compared to intervention patients; median mean daily dose 0.26 vs 0.15 mg/kg/hr (p=0.03), median cumulative dose 183.0 vs 89.8 mg/kg (p=0.02), and median length of exposure 29 vs 22.5 days (p=0.002). Usual care and intervention group patients received similar mean daily doses of benzodiazepines (median 0.23 vs 0.16 mg/kg/hr; p=0.72). Patients in both groups spent similar amounts of time awake and calm (median 70% vs 66% intubated days; p=0.86) and days to first awake and calm state (9 vs 4 days; p=0.20). Modal pain scores were rarely >4 in either group. Patients in the usual care arm experienced fewer study days with any episode of pain (30% vs 50%; p=0.004); percentage of days with any episode of agitation was not significantly different between groups. Two days post-decannulation, the usual care group received more opioids (0.32 vs 0.26 mg/kg/hr; p=0.03) and more sedative classes (3 vs 2; p=0.02) than the intervention group.
Fig. 1.
Sedative doses (a) and SBS scores (b) by day around cannulation by treatment group, and sedative doses (c) and SBS scores (d) by day around decannulation by treatment group.
I = intervention group; NMB = neuromuscular blockade entire day; SBS = State Behavioral Scale; U = usual care group.
During RESTORE’s acute phase of illness, intervention group patients supported on ECMO (n=29) received higher mean daily doses of both opioids (median 0.23 vs 0.10 mg/kg/hr; p<0.001) and benzodiazepines (0.28 vs 0.09 mg/kg/hr; p<0.001) and received more classes of sedatives (3 vs 2; p<0.001) than intervention group patients not on ECMO (n=462). Further, during the acute phase, intervention ECMO patients more commonly received NMB for at least one entire day than intervention non-ECMO patients (83% vs 45%; p<0.001).
Clinically significant iatrogenic withdrawal occurred more frequently in ECMO than non-ECMO patients (27% vs 10%; p<0.001; Table 4), and no age-based differences in clinically significant IWS were noted. No differences were found in median PICU (27.9 vs 28.8 days, p=0.62) or hospital length of stay (36 vs 38 days, p=0.73) between ECMO survivors with and without IWS.
Table 4.
Iatrogenic Withdrawal Syndrome According to Group
| Variables | All Patients | Usual Care Group | Intervention Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ECMOa (n = 41) | No ECMOa (n = 1194) | pb | ECMO (n = 23) | No ECMO (n = 612) | pb | ECMO (n = 18) | No ECMO (n = 582) | pb | |
| WAT-1 score ever ≥3, n/total (%)c | 31/35(89) | 596/840 (71) | 0.007 | 18/20 (90) | 300/409 (73) | 0.02 | 13/15 (87) | 296/431 (69) | 0.14 |
| Clinically significant iatrogenic withdrawal syndrome, n (%)d | 11 (27) | 124 (10) | <0.001 | 8 (35)c | 43 (7) | <0.001 | 3 (17)c | 81 (14) | 0.66 |
ECMO = extracorporeal membrane oxygenation, WAT-1 = Withdrawal Assessment Tool–Version 1.
The ECMO group excludes 20 ECMO patients who were not decannulated prior to study discharge. For the ECMO group, WAT-1 assessments and clinically significant iatrogenic withdrawal events post-decannulation were considered. For the no ECMO group, WAT-1 assessments and events on any study days were considered.
P values for the comparison between groups were calculated using logistic regression accounting for PICU as a cluster variable using generalized estimating equations.
WAT-1 scores ≥3 are associated with clinically significant withdrawal symptoms; calculated for 875 survivors who completed weaning from ≥5 days of opioids and had at least 1 WAT-1 assessment.
Rescue therapy to manage increasing WAT-1 scores.
Discussion
Here we describe sedation management in children supported on ECMO for acute respiratory failure. Our data demonstrate the unique, dynamic, and challenging clinical paradigm presented by pediatric patients with moderate or severe PARDS supported on ECMO. We quantify the trajectory of ECMO sedation management and report a significant increase in opioid and benzodiazepine dosing post-cannulation, continued dose escalation throughout the duration of ECMO support, and development of IWS post-decannulation. Although 30% of ECMO patients are awake and calm on the day of decannulation, the vast majority of ECMO patients remained deeply sedated and/or received neuromuscular blockade throughout their ECMO run. Post-decannulation, nearly 90% of ECMO patients exhibited symptoms of iatrogenic withdrawal and over a quarter required rescue treatment of IWS. Our data also show that a nurse-managed sedation protocol may help limit total sedative exposure in these patients.
In young children, the large ECMO cannulas and their precise positioning requires a calm patient. Although this may require the use of large doses of sedatives, the mechanism for increased sedative requirements during ECMO is likely multifactorial. Although extraction of medications by the ECMO circuit is well-described(8, 10–12), additional factors including expanded volume of distribution and blood administration can alter medication clearance and bioavailability. Patient-specific alterations in organ function resulting from increased perfusion may affect pharmacokinetics and pharmacodynamics of sedatives. The use of RRT during ECMO may also contribute to increased medication requirements.
Our findings support previous data, including the need for a rapid increase in sedative dosing following ECMO cannulation to achieve sedation targets(12, 24). In the first three days post-cannulation, most ECMO patients in our study received neuromuscular blockade and/or were heavily sedated. We found that opioid and benzodiazepine doses increased by 36% and 58%, respectively. By the time of decannulation, ECMO patients were receiving more than double their pre-ECMO doses of these medications for a lighter level of sedation.
Recent data on ECMO management have challenged the use of deep sedation and neuromuscular blockade and emphasize need for early rehabilitation especially in those bridging to lung transplantation on ECMO(25). However, we demonstrate that deep sedation and neuromuscular blockade remains common in patients with acute hypoxemic respiratory failure receiving ECMO. This finding was unrelated to center ECMO volume, suggesting that it may be related to severity of illness of the patients in our cohort.
We demonstrate the effect of discontinuing ECMO on sedation requirements and levels of sedation. Discontinuation of the ECMO circuit and neuromuscular blockade, and reduced sedation levels goals post-decannulation decreased sedative use(5, 6, 8, 9). Within 3 days of decannulation, patients had shifted to a more awake state, and by the fourth day, half of the patients were successfully extubated.
The diagnosis of physiological tolerance, defined as a decreasing clinical effect of a sedative after prolonged exposure, is difficult to operationalize in patients supported on ECMO(26). However, the continued escalation of doses following cannulation and the prevalence of IWS post-decannulation suggests that tolerance occurs more frequently than previously appreciated(13, 14). Specifically, the ECMO circuit is not protecting the patient from increased sedative exposure during ECMO support; a situation that places them at high risk for IWS post-decannulation. In our cohort, patients on ECMO experienced IWS more frequently than comparable patients not supported on ECMO. Both increased exposure to sedatives and the resulting increase in withdrawal are of concern, as data suggests exposure to sedatives and IWS impairs long-term neurodevelopmental outcomes(3, 27).
When comparing patients supported on ECMO in the RESTORE intervention group to those in the usual care group, patients in the intervention group were exposed to less opioids during the study period, despite similar sedative exposure prior to cannulation. Further, intervention patients experienced 6.5 fewer total sedative exposure days. These data suggest that the exposure to opioids and benzodiazepines on ECMO can be mitigated with the use of a standardized, goal-directed, nurse-driven sedation protocol. This must be weighed against the finding that the usual care ECMO patients experienced fewer days with episodic pain compared to intervention patients.
There are several limitations to this study. First, unmeasured variables may have influenced clinical decision-making. Second, as a subset of a larger study, this analysis was not sufficiently powered to answer all questions of interest. Third, we attempted to compare ECMO patients to patients not requiring ECMO who had similar severity of lung disease. Although both groups consisted of patients with moderate or severe PARDS, there may have been unmeasured physiologic or metabolic alterations in patients that contributed to the differences identified. Fourth, we were unable to characterize the full ECMO course for 10 patients who were still on ECMO support at the end of the study period. Nonetheless, the rapid increase in sedative dosing following cannulation and decrease in the days following decannulation suggest that interaction between the ECMO circuit and drug pharmacokinetics plays a clinically significant role in patients supported by ECMO.
Despite these limitations, our findings build on existing research(9, 12, 24) and provide granular data that explores the dynamic patient-ECMO-sedation interactions experienced during ECMO. Further, we demonstrate a clear association with the use of ECMO and the development of IWS. Lastly, our data demonstrate the potential benefit of a nurse-managed protocolized approach to sedation for ECMO patients which deserves future prospective investigation.
Conclusion
The provision of ECMO in children is associated with deep sedation, substantial sedative exposure, and an increased incidence of IWS. It is incumbent upon the pediatric ECMO community to evaluate sedation practices that may be potentially harmful. Standardized, goal-directed, nurse-driven sedation protocols may help mitigate these effects.
Footnotes
No reprints will be requested.
Conflicts of Interest and Source of Funding: No conflicts of interest. Supported by grants from the National Heart, Lung, and Blood Institute and the National Institute of Nursing Research, National Institutes of Health (U01 HL086622 to Dr. Curley and U01 HL086649 to Dr. Wypij).
Copyright form disclosure: Drs. Schneider, Asaro, Wypij, and Curley received support for article research from the National Institutes of Health (NIH). Dr. Schneider’s institution received funding from the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Nursing Research, and the NIH (U01 HL086622 to Dr. Curley and U01 HL086649 to Dr. Wypij). Dr. Asaro’s institution received funding from the NIH/NHLBI. Dr. Wypij’s institution received funding from the NIH/NHLBI. Dr. Thiagarajan’s institution received funding from Bristol Myers Squibb and Pfizer. Dr. Curley’s institution received funding from the NHLBI. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Playfor S, Jenkins I, Boyles C, et al. Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Med. 2006;32:1125–1136. doi: 10.1007/s00134-006-0190-x. [DOI] [PubMed] [Google Scholar]
- 2.Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000;28:2122–2132. doi: 10.1097/00003246-200006000-00079. [DOI] [PubMed] [Google Scholar]
- 3.Davidson A, Flick RP. Neurodevelopmental implications of the use of sedation and analgesia in neonates. Clin Perinatol. 2013;40:559–573. doi: 10.1016/j.clp.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Curley MA, Wypij D, Watson RS, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: A randomized clinical trial. JAMA. 2015;313:379–389. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan O, Klein J, Bohn D, et al. Effects of extracorporeal membrane oxygenation on morphine pharmacokinetics in infants. Crit Care Med. 1994;22:1099–1101. doi: 10.1097/00003246-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Dagan O, Klein J, Gruenwald C, et al. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993;15:263–266. doi: 10.1097/00007691-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Ahsman MJ, Hanekamp M, Wildschut ED, et al. Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin Pharmacokinet. 2010;49:407–419. doi: 10.2165/11319970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Mulla H, Lawson G, Von Anrep C, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000;15:21–26. doi: 10.1177/026765910001500104. [DOI] [PubMed] [Google Scholar]
- 9.Mulla H, Lawson G, Woodland E, et al. Effects of neonatal extracorporeal membrane oxygenation circuits on drug disposition. Current Therapeutic Research. 2000;61:838–848. [Google Scholar]
- 10.Bhatt-Meht V, Annich G. Sedative clearance during extracorporeal membrane oxygenation. Perfusion. 2005;20:309–315. doi: 10.1191/0267659105pf827oa. [DOI] [PubMed] [Google Scholar]
- 11.Wildschut E, Ahsman M, Allegaert K, et al. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109–2116. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulla H, Lawson G, Peek GJ, et al. Plasma concentrations of midazolam in neonates receiving extracorporeal membrane oxygenation. ASAIO Journal. 2003;49:41–47. doi: 10.1097/00002480-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Franck LS, Vilardi J, Durand D, et al. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. American Journal of Critical Care. 1998;7:364. [PubMed] [Google Scholar]
- 14.Arnold JH, Truog RD, Orav EJ, et al. Tolerance and dependence in neonates sedated with fentanyl during extracorporeal membrane oxygenation. Anesthesiology. 1990;73:1136–1140. doi: 10.1097/00000542-199012000-00011. [DOI] [PubMed] [Google Scholar]
- 15.DeBerry BB, Lynch JE, Chernin JM, et al. A survey for pain and sedation medications in pediatric patients during extracorporeal membrane oxygenation. Perfusion. 2005;20:139–143. doi: 10.1191/0267659105pf801oa. [DOI] [PubMed] [Google Scholar]
- 16.Curley MA, Harris SK, Fraser KA, et al. State behavioral scale (SBS) A sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatric Critical Care Medicine. 2006;7:107. doi: 10.1097/01.PCC.0000200955.40962.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franck LS, Harris SK, Soetenga DJ, et al. The withdrawal assessment tool-version 1 (WAT-1): An assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatric Critical Care Medicine. 2008;9:573. doi: 10.1097/PCC.0b013e31818c8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–297. [PubMed] [Google Scholar]
- 19.Wong DL, Baker CM. Pain in children: Comparison of assessment scales. Pediatr Nurs. 1988;14:9–17. [PubMed] [Google Scholar]
- 20.Solodiuk J, Curley MA. Pain assessment in nonverbal children with severe cognitive impairments: The individualized numeric rating scale (INRS) J Pediatr Nurs. 2003;18:295–299. doi: 10.1016/s0882-5963(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 21.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 22.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric acute respiratory distress syndrome: Definition, incidence, and epidemiology: Proceedings from the pediatric acute lung injury consensus conference. Pediatric Critical Care Medicine. 2015;16:S23–40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 24.Shekar K, Roberts JA, Mullany DV, et al. Increased sedation requirements in patients receiving extracorporeal membrane oxygenation for respiratory and cardiorespiratory failure. Anaesth Intensive Care. 2012;40:648. doi: 10.1177/0310057X1204000411. [DOI] [PubMed] [Google Scholar]
- 25.Turner DA, Rehder KJ, Bonadonna D, et al. Ambulatory ECMO as a bridge to lung transplant in a previously well pediatric patient with ARDS. Pediatrics. 2014;134:e583–5. doi: 10.1542/peds.2013-3435. [DOI] [PubMed] [Google Scholar]
- 26.Anand KJ, Willson DF, Berger J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125:e1208–25. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madderom MJ, Reuser JJ, Utens EM, et al. Neurodevelopmental, educational and behavioral outcome at 8 years after neonatal ECMO: A nationwide multicenter study. Intensive Care Med. 2013;39:1584–1593. doi: 10.1007/s00134-013-2973-1. [DOI] [PubMed] [Google Scholar]