Figure 3. Therapeutic activity of ENDOS/ADC against SJSA-1 osteosarcoma xenografts.
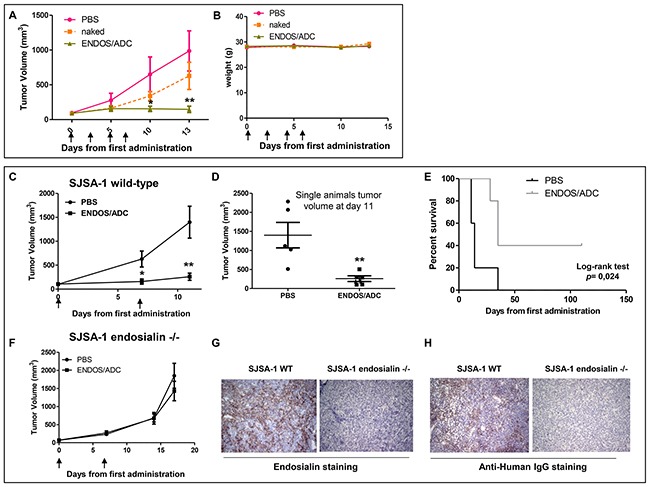
(A) Mice were implanted subcutaneously with 2×106 SJSA-1 cells. When tumors reached a volume of ∼100 mm3, mice were randomly grouped (N=5) and intravenously injected every 3 days with vehicle (PBS), naked hMP-E-8.3 antibody (10 mg/kg), or ENDOS/ADC (10 mg/kg) for a total of 4 injections. (B) No significant treatment-related body weight loss was registered. (C) Growth curves of SJSA-1 tumor xenografts treated with a reduced schedule (two injections of 10 mg/kg ENDOS/ADC one week a part). (D) Plot of single animals tumor volumes at day 11th. (E) Survival curve evaluated by Kaplan-Meier and analyzed by the log-rank test. (F) Growth curves of endosialin −/− SJSA-1 derived xenografts treated as in C. IHC staining of endosialin (G) or ENDOS/ADC (H) in wild-type or endosialin −/− SJSA-1 derived xenografts. * p < 0.05, ** p < 0.01.
