Table 2.
AlCl3/Al promoted‐carboxylation of benzene derivatives.

| Reactant | T [°C] | Main product | Yield[a] [%] | Selectivity [% o/m/p] |
|---|---|---|---|---|
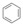
|
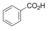
|
|||
| 26 a | 70 | 27 a | 88 | – |
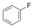
|
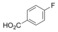
|
|||
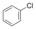
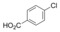
| 26 b | 40 | 27 b | 47 | 1/1/98 |
|
|
|||
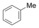
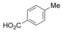
| 26 c | 60 | 27 c | 45 | 3/2/95 |
|
|
|||
| 26 d | 70 | 27 d | 69 | 7/3/90 |
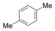
|
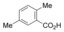
|
|||
| 26 e | 40 | 27 e | 92 | – |
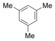
|
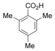
|
|||
| 26 f | 20 | 27 f | 80[b] | – |
[a] Yields are based on the quantity of AlBr3. [b] 68 h reaction time.
