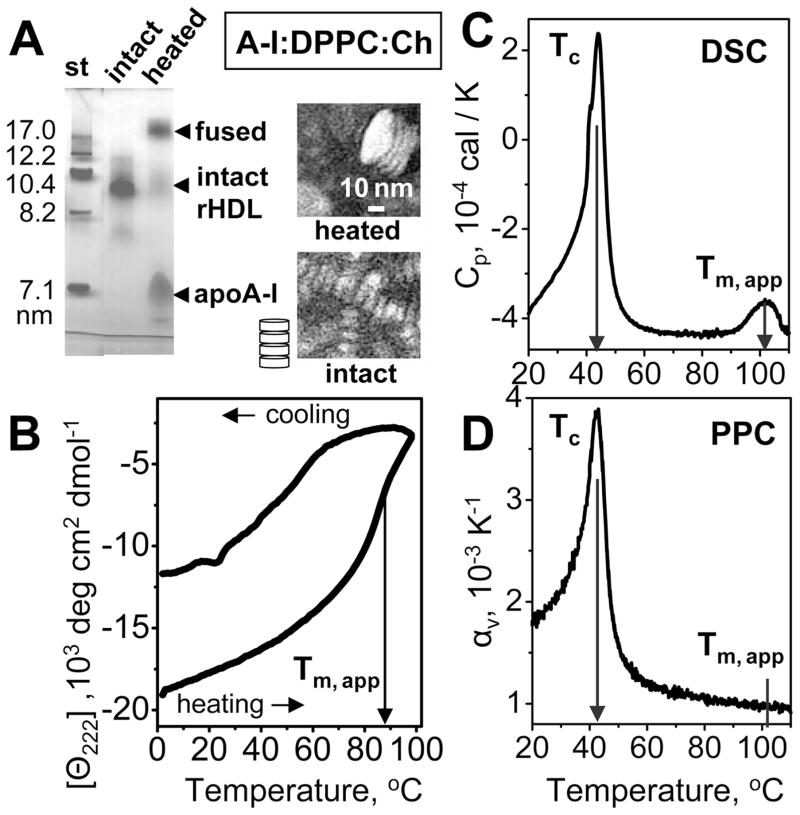
Figure 4.
Heat-induced remodeling of discoidal rHDL reconstituted from human apoA-I, DPPC, and cholesterol. (A) Nondenaturing gel electrophoresis and negative stain EM of rHDL that were intact or heated to 110 °C and cooled to 22 °C. NDGE shows that such heating leads to irreversible remodeling of intact rHDL (d ~ 9.6 nm) into dissociated apoA-I and protein-containing vesicles (d ≥ 25 nm). EM shows intact rHDL as discoidal particles with 〈d〉 = 9.6 nm stacked on edge (illustrated in a cartoon), which is characteristic of the negative stain preparations. EM of the heated particles shows stacks of collapsed SUVs (d ~ 30 nm) that are products of rHDL fusion. (B) Thermal denaturation of rHDL monitored by CD at 222 nm for α-helical unfolding. The samples (0.02 mg/mL protein in 10 mM sodium phosphate, pH 7.5) were heated and cooled from 5 to 98 °C at a rate of 11 °C/h. The apparent melting temperature (Tm,app) corresponding to the inflection point in the heating curve is indicated. (C) DSC data of rHDL (1 mg/mL protein in standard buffer) were collected upon heating at a rate of 90 °C/h. Tc indicates the peak temperature of the chain melting transition in DMPC. The peak at Tm,app corresponds to thermal denaturation of rHDL. (D) PPC data of rHDL recorded under conditions similar to those used to record the DSC data. The chain melting transition in DPPC is observed at Tc; no peak in αv(T) is detected near Tm,app.