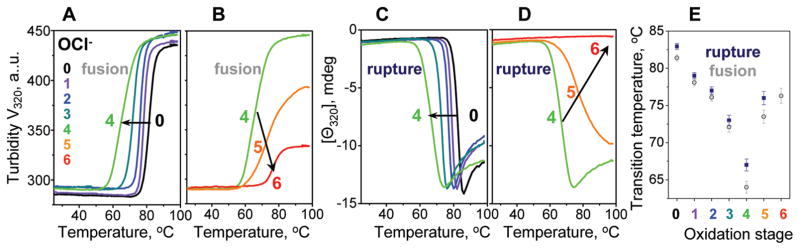
Figure 4.
Effects of oxidation on the apparent thermal stability of VLDL. VLDL2 that were intact (0) or oxidized by OCl− to stages 1–6 were diluted to 0.1 mg/mL protein in standard buffer and heated at a rate of 11 °C/h. Increase in the particle size due to VLDL fusion followed by rupture and coalescence into lipid droplets was monitored by turbidity at 320 nm (A, B), which is proportional to the dynode voltage V320 measured in CD experiments (47). Repacking of apolar core lipids upon VLDL rupture and coalescence into lipid droplets was monitored by CD at 320 nm, Θ320(T) (C, D), that was measured simultaneously with V320(T) (A, B). Arrows indicate changes in the heating data upon progressive oxidation. (E) The apparent transition temperatures, which were determined from the peak positions in the first derivatives of the melting data, dV320(T)/dT (for fusion and rupture) and dΘ320(T)/dT (for rupture), are plotted as a function of oxidation stage. Error bars reflect accuracy in Tm determination.