FIG 2.
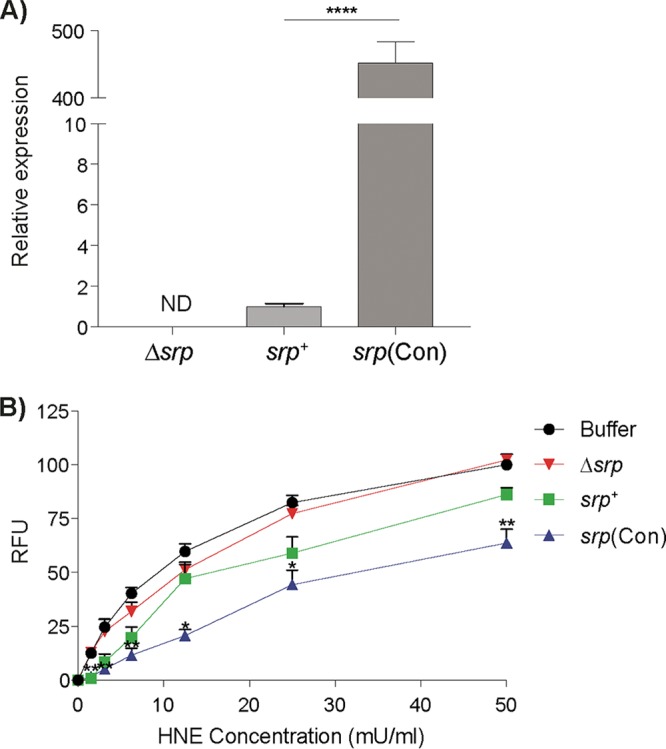
B. longum constitutively expressing srp inhibits human neutrophil elastase activity in vitro. (A) srp mRNA levels were quantified from various B. longum strains. Expression of srp was higher in B. longum srp(Con) than in B. longum srp+ (****, P < 0.0001). No srp mRNA was detected in B. longum Δsrp (n = 4). (B) Inhibitory capacities of various B. longum strains were tested in vitro, as measured by fluorescence produced via cleavage of FITC-elastin substrate at 1.5, 3.125, 6.25, 12.5, 25, and 50 mU/ml, expressed as relative fluorescence units (RFU) (n = 3/group). Compared to B. longum Δsrp, B. longum srp+ inhibited cleavage of elastin by human neutrophil elastase (HNE) in vitro at 1.5 mU/ml (P < 0.01), resulting in lower RFU. In the same assay, B. longum srp(Con) further inhibited HNE across all concentrations of HNE compared to B. longum Δsrp, resulting in lower RFU. As well, B. longum Δsrp did not inhibit HNE, as the levels of cleavage of FITC-elastin determined by RFU produced were not significantly different between B. longum Δsrp and buffer alone at any concentration of HNE added. Buffer, HNE alone; srp+, B. longum srp+; Δsrp, B. longum Δsrp; srp(Con), B. longum srp(Con). ND, not detectable. Data are shown as means ± SEM. Statistical significance determinations were performed using the Kruskal-Wallis test. *, P < 0.05 (versus B. longum Δsrp); **, P < 0.01 (versus B. longum Δsrp).
