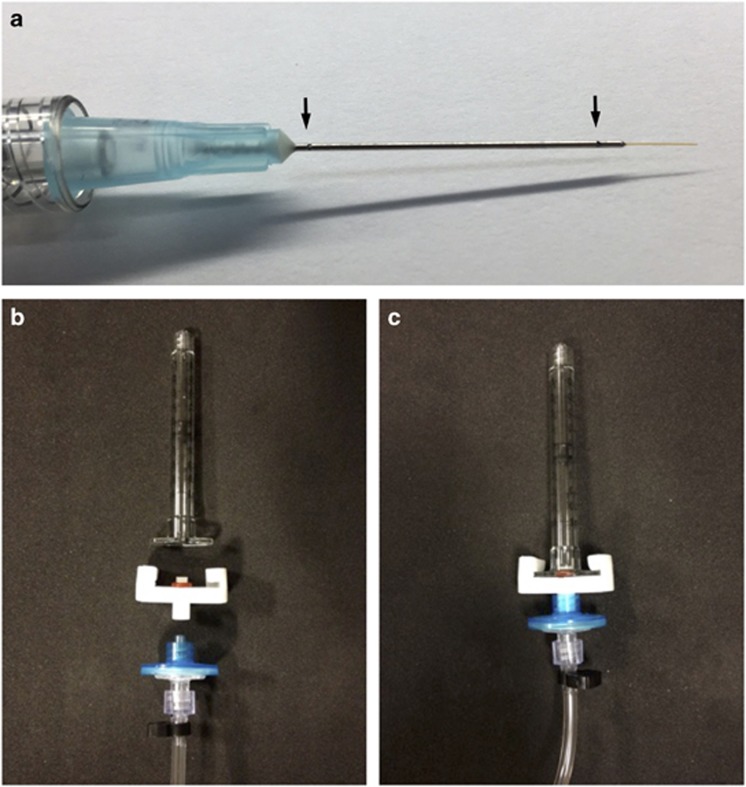
Figure 1.
Subretinal gene therapy injection system. (a) A dual-bore 41 G blunt polytetrafluoroethylene (Teflon) tipped cannula mounted within a 23 G steel shaft (DORC, Zuidland, The Netherlands). Arrows indicate proximal and distal pressure relief holes along the shaft, which allow evacuation of excess vitreous fluid out of the eye during the injection. (b) Components of the subretinal injection system consisting of a 1 ml BD syringe, custom syringe lock with a rubber O-ring, air filter and polyethylene connection tubing (Alchimia, Ponte S Nicolò, Italy). (c) Assembled injection system.