Table 2. Exploration of the 5-Position of the Pyrazine Ring.
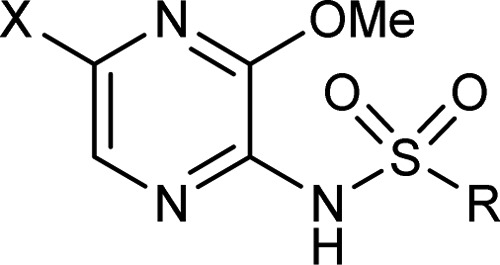
| example | R | X | CCR4 pIC50a |
|---|---|---|---|
| 1 | 5-chloro-2-thienyl | Br | 7.2 ± 0.2 |
| 2 | 2-thienyl | Br | 6.2 ± 0.2 |
| 3 | 3-thienyl | Br | 5.9 ± 0.1 |
| 4 | 4,5-dichloro-2-thienyl | Br | 6.0 ± 0.1 |
| 5 | 3-bromo-5-chloro-2-thienyl | Br | 7.4 ± 0.2 |
| 6 | 3-phenyl-2-thienyl | Br | 6.0 ± 0.1 |
| 7 | phenyl | Br | 6.1 ± 0.1 |
| 8 | 2-chlorophenyl | Br | 6.8 ± 0.2 |
| 9 | 3-chlorophenyl | Br | 7.0 ± 0.2 |
| 10 | 4-chlorophenyl | Br | 6.9 ± 0.2 |
| 11 | 2,6-dichlorophenyl | Br | 6.2 ± 0.2 |
| 12 | 2,5-dichlorophenyl | Br | 5.5 ± 0.1 |
| 13 | 2,4-dichlorophenyl | Br | 6.9 ± 0.2 |
| 14 | 3,5-dichlorophenyl | Br | 5.3 ± 0.1 |
| 15 | 3,4-dichlorophenyl | Br | 6.7 ± 0.2 |
| 16 | 2,3-dichlorophenyl | Br | 8.0 ± 0.2 |
| 17 | 2,3-dichlorophenyl | Cl | 7.9 ± 0.2 |
| 18 | 3,4-dichloro-2-thienyl | Cl | 8.1 ± 0.2 |
| 19 | 2-chloro-3-fluorophenyl | Cl | 7.8 ± 0.2 |
| 20 | 3-chloro-2-fluorophenyl | Cl | 7.1 ± 0.2 |
| 21 | 3-chloro-2-methylphenyl | Cl | 7.7 ± 0.2 |
| 22 | 2-chloro-3-(trifluoromethyl)phenyl | Cl | 7.2 ± 0.1 |
| 23 | 3-chloro-2-cyanophenyl | Cl | 7.2 ± 0.2 |
| 24 | 3-chloro-2-methylthiophenyl | Cl | 7.1 ± 0.2 |
| 25 | 2,3-dichloro-4-pyridyl | Cl | 5.0 ± 0.1 |
| 26 | butyl | Cl | 5.5 ± 0.2 |
Potency is given as pIC50 values with n = ≥ 2 replicates.
