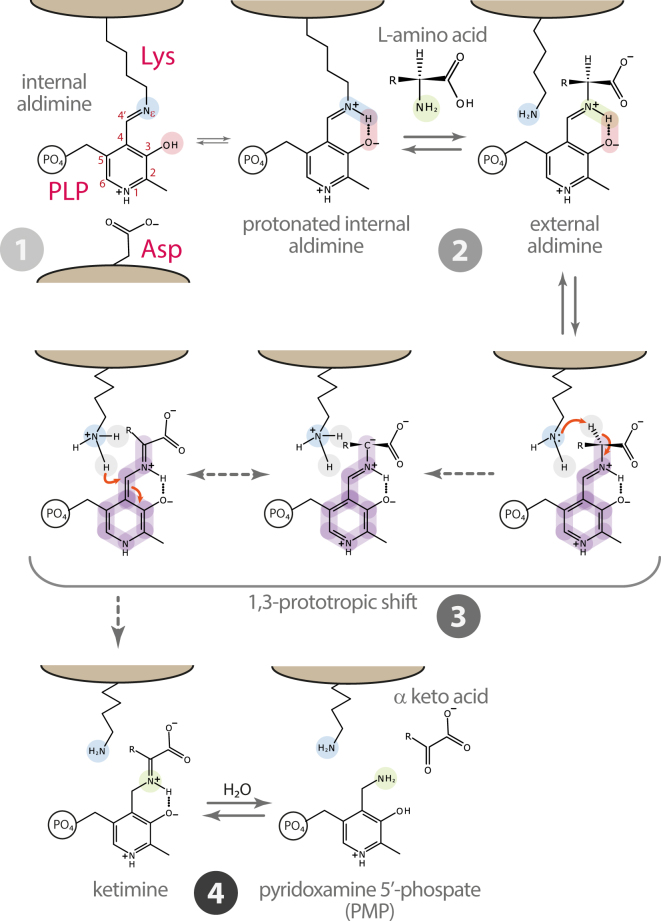
Figure 1.
General mechanism for the first half reaction of aminotransferases. (1) A conserved Asp residue protonates the pyridine nitrogen of PLP (N1). (2) The transaldimination reaction with the amino acid substrate occurs only when the internal aldimine is protonated at Nε. (3) The 1,3-prototropic shift represents the rate limiting step of the half reaction. The amino group of the Lys residue acts as a base catalyst extracting the Cα hydrogen. According to the Dunathan hypothesis, co-planarity of the Schiff base bond of the external aldimine with the pyridine plane forms an extended π-system that, together with protonation of N1, allows the negative charge on Cα carbanion to be stabilized by a combination of Coulombic and resonance effects3. The same Lys residue serves as an acid catalyst in the protonation of C4′ completing the 1,3-proton transfer. (4) Hydrolysis of the resulting ketimine yields pyridoxamine phosphate (PMP) and the leaving α-keto acid.