Abstract
Objective:
To study the role of advanced applications of digital mammogram, whether contrast-enhanced spectral mammography (CESM) or digital breast tomosynthesis (DBT), in the “T” staging of histologically proven breast cancer before planning for treatment management.
Methods:
In this prospective analysis, we evaluated 98 proved malignant breast masses regarding their size, multiplicity and the presence of associated clusters of microcalcifications. Evaluation methods included digital mammography (DM), 3D tomosynthesis and CESM. Traditional DM was first performed then in a period of 10–14-day interval; breast tomosynthesis and contrast-based mammography were performed for the involved breast only. Views at tomosynthesis were acquired in a “step-and-shoot” tube motion mode to produce multiple (11–15), low-dose images and in contrast-enhanced study, low-energy (22–33 kVp) and high-energy (44–49 kVp) exposures were taken after the i.v. injection of the contrast agent. Operative data were the gold standard reference.
Results:
Breast tomosynthesis showed the highest accuracy in size assessment (n = 69, 70.4%) than contrast-enhanced (n = 49, 50%) and regular mammography (n = 59, 60.2%). Contrast-enhanced mammography presented the least performance in assessing calcifications, yet it was most sensitive in the detection of multiplicity (92.3%), followed by tomosynthesis (77%) and regular mammography (53.8%). The combined analysis of the three modalities provided an accuracy of 74% in the “T” staging of breast cancer.
Conclusion:
The combined application of tomosynthesis and contrast-enhanced digital mammogram enhanced the performance of the traditional DM and presented an informative method in the staging of breast cancer.
Advances in knowledge:
Staging and management planning of breast cancer can divert according to tumour size, multiplicity and the presence of microcalcifications. DBT shows sharp outlines of the tumour with no overlap tissue and spots microcalcifications. Contrast-enhanced spectral mammogram shows the extent of abnormal contrast uptake and detects multiplicity. Integrated analysis provides optimal findings for proper “T” staging of breast cancer.
INTRODUCTION
Breast imaging sometimes presents a challenge for the diagnostic performance of traditional mammography, especially those with dense glandular tissues.1 Digital systems offer the potential to improve sensitivity.2 However, tissue overlap could be displayed between the normal soft tissues of the breast, tumours and calcium deposits, and so decreases the conspicuity of breast lesions. Even when tumours are detected, the full extent of disease may not be clearly depicted.3
Breast tomosynthesis is an application of digital mammogram which can overcome the problem of tissue overlap especially in dense breasts.
Promising results added to the body of literature4,5 on the potential impact of digital breast tomosynthesis (DBT) in increasing the sensitivity in breast cancer screening and reducing the call-back rate. Detection of additional suspicious lesions in tomosynthesis would enable the surgeon to prepare the patient for a more radical surgery than originally planned.6
Contrast-enhanced MRI is currently the most sensitive breast cancer detection technique, but may have high false-positive rates, higher costs and lower availability.7 Contrast-enhanced digital mammography is a new alternative; with the injection of the contrast medium, it is able to depict angiogenesis in breast carcinoma.8 The use of contrast-enhanced digital mammography in the staging of breast cancer is still under investigation.
This work presents the initial performance in studying the role of advanced applications of digital mammogram: contrast-enhanced spectral mammography (CESM) and DBT in the staging of breast cancer.
METHODS AND MATERIALS
Patients
The present work is a prospective analysis that included 98 female patients; their age ranged from 26 to 70 years and the mean age was 50.2 ± 10.6 years (mean ± standard deviation). The study duration was 18 months starting from December 2013 till June 2015.
Inclusion criteria: histologically proved malignant breast masses (Breast Imaging Reporting and Data System 6) that were first diagnosed by open or core biopsy. Cases selected are those having one or all of the following: (1) a dense breast on mammogram, (2) positive family history of breast cancer and (3) those known to have previous precancerous breast lesions, since they are more susceptible to locally advanced or multicentric carcinomas.
All the patients were first subjected to traditional digital mammogram to detect the breast cancer. For benefits of proper staging and choice of management options, further imaging with the advanced applications of digital mammogram (DBT and CESM) was requested to these selected cases.
Exclusion criteria: (1) proved benign masses detected on mammogram; (2) malignant-looking or suspicious masses with unavailable pathology report; (3) patients who are pregnant; and (4) contrast media contraindication of injection as in renal impairment (with a glomerular filtration rate (GFR) >30 ml min−1/1.73 m2) or history of allergy.
Ethical approval
The study was approved by the ethics committee of the Scientific Research Review Board of the Radiology Department, National Cancer Institute, Cairo University.
Patients included had given informed consent to use their data in research studies.
Radiation safety committee approval was obtained by the Biomedical Engineering Department, Cairo University.
Methods
Included cases were imaged with digital mammography (DM), DBT and contrast dual-energy spectral enhanced mammography performed on a Senographe Essential full-field DM system (GE Healthcare, Chalfont St-Giles, UK).
Both breasts were imaged in the craniocaudal and mediolateral oblique views by digital mammogram. Only the involved breast (the one with cancer) is imaged by both tomosynthesis and contrast-enhanced mammography in the same session with a time interval of 10–15 days from the tradition digital mammogram. In our institute, the process of confirmation of malignancy passing by a medicolegal approval for biopsy, biopsy performance and finally the analysis of the biopsied tissue lasts for 5–7-day durations. Then, proved cases of breast cancer are reviewed by a weekly tumour board committee of consultants in breast cancer surgery, radiology and clinical oncology.
Digital tomosynthesis
Tomosynthesis examinations were performed in the same views with breast compression as traditional (two-dimensional) DM. Standard views were acquired in a “step-and-shoot” tube motion mode to produce multiple (11–15), low-dose images of the breast acquired at different angles, while the X-ray tube moves in an arc around the compressed breast. The settings were: 36 mA, kilovoltage ranging from 22 kV to 25 kV, with “automatic optimization of parameters” used to ensure appropriate anode, filter, kilovoltage and milliampere second that vary according to the breast density and thickness in order to get the optimal image quality and radiation dose. These images were then used to reconstruct a series of 1-mm-thick images (from 60 to 90 slices, depending on breast thickness). The used device for breast tomosynthesis is powered by “ASiRDBT”, which is an iterative reconstruction algorithm, that provides full filed digital mammography-like images and enhances the conspicuity of microcalcification. The reconstructed tomosynthesis images can be viewed as one slice at a time or in a cine loop.
Contrast-enhanced spectral mammography
In this procedure, an iodinated contrast agent (iohexol, 300 mg I ml−1) was injected manually through a catheter introduced in the antecubital vein at a dose of 1.5 ml kg−1 before application of compression to avoid interference with the normal blood passage to the breast. After a delay of 2 min, images were taken in the standard mammography images. Low- and high-energy exposures were consecutively performed in each view during a single compression to minimize motion artefact. Low-energy images simulate those of standard mammography and were acquired at peak kilovoltage values ranging from 26 kVp to 31 kVp, which is below the k-edge of iodine. High-energy images were acquired at 45–49 kVp, which is above the k-edge of iodine. At high-energy image areas, there is enhancement of areas of contrast uptake.
Image post-processing was then performed by a weighted logarithmic subtraction of the two images (i.e. low and high energy) to display an iodine-enhanced image, where the density of the normal glandular tissue was reduced and areas of contrast uptake became accentuated.
Image analysis
Four readers assessed all cases in individual sessions using Image Diagnostic Mammography Workstation (GE Healthcare) as follows.
DM and DBT findings were analyzed by two independent readers with 20 and 18 years' experience in breast imaging. The type of findings depicted on mammography and DBT slices were interpreted for the extent of the borders of the included masses and focal architectural distortion ± segmental/clustered microcalcifications.
CESM images, whether low- or high-energy images, were evaluated by other two individual readers, both with 25 years' experience in breast imaging.
Subtracted CESM images were reviewed for enhancing masses or focal areas of abnormal contrast uptake. For evaluation of non-enhancing lesions and microcalcifications, low-energy images were the source.
Points of correlation between mammogram applications were:
(i) size: the largest dimension of the dominant mass obtained by a virtual ruler tool on the desktop of the used modalities
(ii) multiplicity
(iii) associate clusters of microcalcifications: assessed for the presence of related focal distortion on the tomosynthesis and enhancement on the contrast-enhanced mammography images.
Then, all of the four readers did a combined analysis; one time DBT with CESM and another time the three applications all together (DBT, CESM and DM).
The choice of the surgical procedure diverts between cases and was selected according to the modality that showed the worst stage of breast cancer: (1) breast conservative surgery of focal lumpectomy with sentinel lymph node biopsy (n = 22) in case of unifocal tumour, and the axillary nodes were average sized and benign looking, or lumpectomy and axillary clearance (n = 39), if there were pathologically enlarged nodes; (2) quadrantectomy with axillary clearance (n = 14) for large unifocal (>5 cm) or multifocal tumours; (3) partial mastectomy with axillary clearance and skin sparing (n = 15) in case of multicentric tumours; (4) radical mastectomy without skin sparing (n = 8) in case the intervening distance between the tumour and the skin is <5 mm or if the cancer is associated with marked inflammatory changes (interstitial oedema, dermal thickening and peau d' orange).
The surgically removed complete pathologic specimens were the gold standard of reference.
Malignant masses removed at surgery were measured in millimetres with a calibrated ruler before being fixed in formalin.
Statistical analysis
The collected data were coded, tabulated and statistically analyzed using a statistical package (SPSS® v. 20; IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). Descriptive statistics were performed for quantitative parametric data as mean ± standard deviation, whereas they were performed for qualitative data as number and percentage. Comparison between categorical data was performed using χ2 test.
Standard diagnostic indices including sensitivity, specificity, positive-predictive value (PPV), negative-predictive value (NPV) and diagnostic accuracy were calculated. Comparisons of sensitivity or specificity between mammography, tomosynthesis and CESM were performed.
The level of statistical significance (p-value) for all tests was set at 0.05.
RESULTS
The present work included 98 proved breast carcinomas. 63 (64.3%) of them were invasive ductal carcinoma, grade 2–3, 17 (17.3%) carcinomas were invasive ductal carcinoma with an intraductal carcinoma in situ component, 14 (14.3%) carcinomas were diagnosed as mixed invasive ductal plus lobular carcinoma and 4 (4.1%) carcinomas were micropapillary carcinoma.
Size (of the largest mass in case of multiple lesions), multiplicity as well as the presence of calcifications were evaluated for “T” staging of the included breast cancer.
Individual diagnostic performance of different digital mammogram applications
Comparison between each breast imaging modality: DM, DBT and CESM results are shown in Table 1.
Table 1.
Distribution of malignant findings and assessments by different digital mammography applications in correspondence with operative pathology specimen
| Variable | Size |
Multiplicity |
Calcifications |
|||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |
| DM | 59 | 60.2 | 7 | 7.1 | 23 | 23.4 |
| DBT | 69 | 70.4 | 10 | 10.2 | 23 | 23.4 |
| CESM | 49 | 50.0 | 12 | 12.2 | 10 | 10.2 |
| Pathology | 98 | 100.0 | 13 | 13.2 | 23 | 23.4 |
CESM, contrast-enhanced spectral mammography; DBT, digital breast tomosynthesis; DM, digital mammography.
Sensitivity of DM in detection of size was 60.2% (n = 59), that of DBT was 70.4% (n = 69) and that of CESM was 50% (n = 49).
Multiplicity of the breast carcinoma was suggested in 13 cases (n = 34 masses with an extra of 21 masses); a significant relationship was found between these multiplicities diagnosed by DM, DBT and CESM vs pathology, and p-values were 0.004, 0.004 and 0.003, respectively.
CESM with the aid of high-energy images was the most sensitive application in detecting satellites. The sensitivity of CESM was 92.3% compared with only 53.8% for DM and 77% for DBT. CESM also showed the highest NPV of 98.7%. The highest specificity was shown by DBT (95.3%) followed by DM (94.1%) and then CESM (91.7%).
DBT and CESM showed near equal accuracy values of 92.8% and 91.8%, respectively. DM presented a less accurate value of 88.7%
Pathological calcifications and focal distortion were detected in 23 cases. DM and DBT were able to detect 100% of these cases (Figure 1) and showed significant association with those detected by pathology with a p-value <0.005.
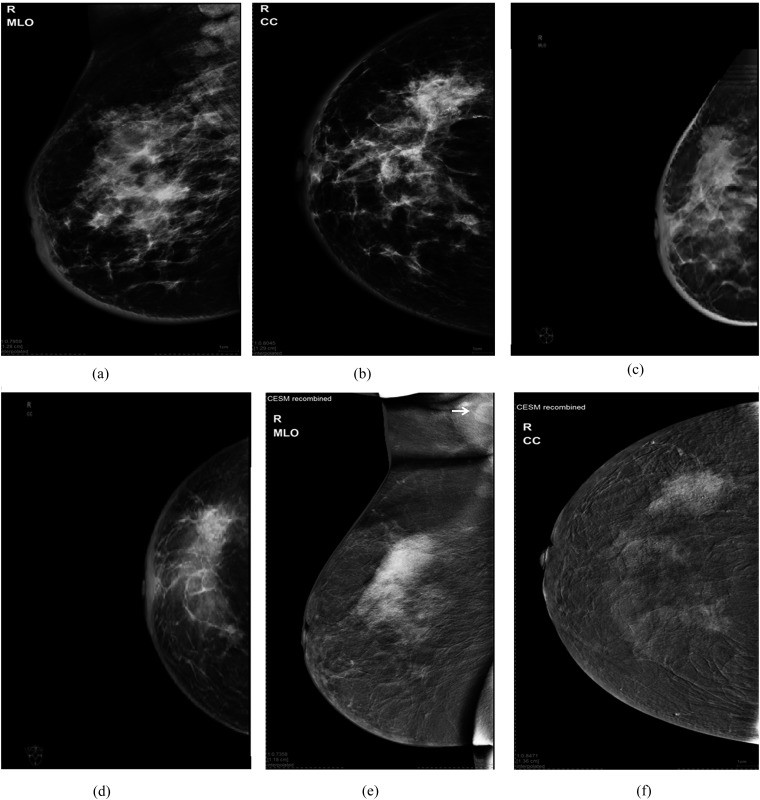
Figure 1.
A 56-year-old female presented with skin oedema, breast induration and enlarged axillary nodes: (a, b) digital mammogram mediolateral oblique (MLO) and craniocaudal (CC) views of the right breast are showing an upper outer mass (maximum dimension: 62 mm) and another deep central one with associated focal distortion and microcalcifications. Also, there is dermal oedema. (c, d) Tomosynthesis MLO and CC views are showing upper outer quadrant (UOQ) mass with ductal extensions (the overall maximum length: 80 mm) into the lower outer quadrant (LOQ) and central region. (e, f) Contrast-enhanced spectral mammography (CESM) MLO and CC views are showing non-mass enhancement (maximum length: 98 mm) of ductal distribution seen in the LOQ and deep central region. A large enhancing axillary lymph node is also noted (arrow in “e”). Histopathology: invasive ductal carcinoma grade 2 with intraductal component of 110-mm maximum length.
In CESM, calcifications were spotted in only 10 cases with the aid of low-energy images, where an associated abnormal enhancement was noted in the subtraction images.
Here, CESM showed the least sensitivity (43.4%), accuracy (86.7%) and NPV (85.2%). All of the included digital mammogram applications displayed a 100% specificity and PPV.
Value of merging different digital mammogram applications in estimation of breast cancer extension, multiplicity and calcifications
Size
Combined evaluation of DM and DBT enhanced the performance of both of these modalities: correct size was estimated in 72 masses (Figure 2), with a sensitivity of 75%, PPV of 100% and accuracy of 75.5% (Table 2). A less convenient diagnostic performance was elicited in the combined evaluation of DM and CESM, where accurate measures were determined in 62 masses and underestimated in 36 masses (Figure 2).
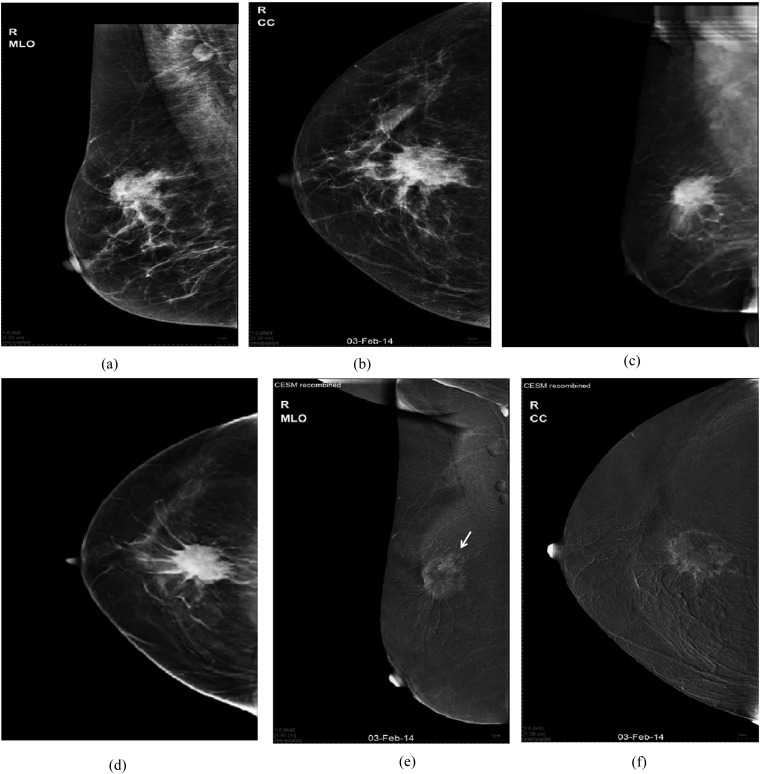
Figure 2.
A 59-year-old female patient presenting with non-tender breast and axillary lumps: (a, b) digital mammogram mediolateral oblique (MLO) and craniocaudal (CC) views of the right breast are showing an upper central spiculated mass (maximum length 70 mm) with enlarged axillary nodes. (c, d) Tomosynthesis MLO and CC views are showing the aforementioned mass with long tentacles extending further than those seen on regular mammogram. We considered these tentacles as part of the extension of the malignant mass and so, there was overestimation of the maximum length of the malignant mass to be 82 mm. (e, f) Contrast-enhanced spectral mammography (CESM) MLO and CC views are showing upper outer mass with rim enhancement (maximum length 66 mm). Another adherent smaller mass was (arrow in “e”) only clarified at the contrast-enhanced images. Histopathology: multifocal invasive ductal carcinoma grade 2. Maximum length of the dominant mass was 66 mm.
Table 2.
Number of lesions assessed for size (maximum dimension) using combined mammography applications vs pathology
| Included breast cancer (n=98) | DM + DBT | DM + CESM | DBT + CESM | DM + DBT + CESM |
|---|---|---|---|---|
| FN | 24 | 36 | 22 | 22 |
| FP | – | – | – | – |
| TN | 2 | – | 3 | 3 |
| TP | 72 | 62 | 73 | 73 |
CESM, contrast-enhanced spectral mammography; DBT, digital breast tomosynthesis; DM, digital mammography; FN, false negative; FP, false positive; TN, true negative; TP, true positive.
Best performance was found with the combined evaluation of CESM and DBT, which estimated the accurate size of 73 (74.5%) masses and the PPV was 100%. The latter values were the same as those also elicited by the combined evaluation of DM, DBT and CESM all together with a sensitivity of 75.5%, PPV of 100% and accuracy of 77.5%.
Multiplicity
Many more lesions were detected by CESM vs DBT (Table 3).
Table 3.
Number of lesions with other multiple foci detected when merging the performance of different mammography applications vs pathology
| Included breast cancer (n=98) | DM + DBT | DM + CESM | DBT + CESM | DM + DBT + CESM |
|---|---|---|---|---|
| FN | 6 | 1 | 1 | 1 |
| FP | 3 | 8 | 10 | 10 |
| TN | 82 | 77 | 75 | 75 |
| TP | 7 | 12 | 12 | 12 |
CESM, contrast-enhanced spectral mammography; DBT, digital breast tomosynthesis; DM, digital mammography; FN, false negative; FP, false positive; TN, true negative; TP, true positive.
The highest sensitivity (92.3%) was found when the data found at CESM were added to those of DM or DBT.
The combined performance of DBT with CESM has shown the highest specificity and PPV of 97.4% and 85.7%, respectively (Figure 3).
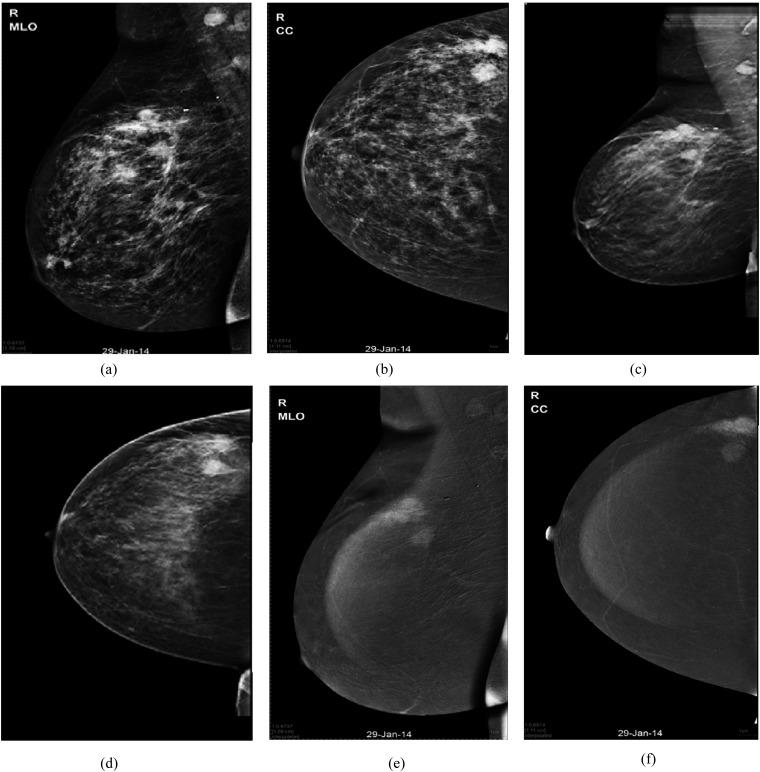
Figure 3.
A 59-year-old female patient with right breast masses and palpable ipsilateral axillary nodes: (a, b) digital mammography mediolateral oblique (MLO) and craniocaudal (CC) views of the right breast are showing upper outer quadrant (UOQ) few indistinct masses associated with malignant-looking microcalcifications (overall maximum length 60 mm) and enlarged ipsilateral axillary nodes. (c, d) Tomosynthesis MLO and CC views are displaying UOQ multifocal pathology and associated microcalcifications (overall maximum length 58 mm). (e, f) Contrast-enhanced spectral mammography MLO and CC views are showing UOQ gathered few spiculated masses of heterogeneous contrast enhancement. Maximum length of the dominant mass was 38 mm. Histopathology: invasive ductal carcinoma grade 2 with comedo ductal carcinoma in situ (maximum length 55 mm).
Calcifications
CESM was not the proper application for assessment of mammary calcifications. The presence of DM in the evaluation compromised the lower performance of CESM (Figures 1 and 3) and provided a sensitivity of 100%, a PPV of 100% and an accuracy of 100% whether adding DM to DBT, or DM to CESM or all of the three modalities (DM + DBT + CESM). Also, adding DBT to CESM upgraded the performance of the latter in the analysis of calcifications.
T staging
The T stage was determined according to the mass index size + speculation, skin and deep muscular extensions and then compared with post-surgical pathology results, as shown in Table 4.
Table 4.
“T” staging by different mammogram modalities
| Stage | DM |
DBT |
CESM |
|||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |
| T1 | 10 | 10.2 | 10 | 10.2 | 13 | 13.2 |
| T2 | 46 | 47 | 49 | 50.0 | 59 | 60.2 |
| T3 | 19 | 19.3 | 16 | 16.3 | 13 | 13.3 |
| T4b | 23 | 23.5 | 23 | 23.5 | 13 | 13.3 |
| Total | 98 | 100 | 98 | 100 | 98 | 100 |
| Pearson correlation | 0.721a |
0.760a |
0.762a |
|||
| Significance (two-tailed) | 0.000 | 0.000 | 0.000 | |||
CESM, contrast-enhanced spectral mammography; DBT, digital breast tomosynthesis; DM, digital mammography.
Correlation is significant at the 0.01 level (two-tailed).
A significant positive correlation was found between pathology “T” staging and staging suggested by each mammography application (DM, DBT and CESM) (p < 0.001).
An accurate “T” staging was determined in 65 (66.3%) carcinomas on DM, 75 (76.5%) carcinomas on DBT and 80 (79.6%) carcinomas on CESM.
CESM is the modality of choice in the assessment of early breast cancer (T1 and T2 stages), as it provided an accurate “T” staging for 72 (73.5%) carcinomas compared with only 56 (57.1%) carcinomas and 59 (60.2%) carcinomas properly staged by DM and DBT (Figures 1 and 2), respectively.
In case of locally advanced breast carcinoma (T3 and T4), DM + DBT was more accurate in decision-making.
The combined analysis of DM + DBT + CESM provided 74% accuracy in “T” staging of breast cancer.
DISCUSSION
Mammography is still the most consistent method for the early detection of breast cancer; yet, the full extent of the disease may not be clearly depicted.1,9
The present study evaluated 98 proven malignant breast masses using DM compared with DBT and CESM image sets as regards the tumour size, multiplicity and the presence of associated pathological microcalcifications (i.e. calcifications with distortion and/or contrast uptake) and finally, the overall “T” staging. Pathology of the surgical specimen was the gold standard of reference.
On individual basis, DBT was most sensitive in estimating the correct size of the included carcinomas with a sensitivity value of 70.4% (n = 69/98), followed by DM (60.2%, n = 59) and then at last CESM (50%, n = 49). All results were statistically significant vs operative findings with a p-value of <0.005. Our results were consistent with the study of Svahn et al,10 which stated that the diagnostic accuracy of DBT was significantly better than that of DM with an average sensitivity of 90% vs 79%.
CESM displayed the highest percentage in the underestimation of the size of the mass (n = 10; 10.2%) and also in the overestimation (n = 39; 39.8%). DM showed overestimation of the size of the mass compared with pathology in 30 (30.6%) masses and underestimation in 9 (9.2%) masses. Regarding DBT, there was no size underestimation; but, on the other hand, overestimation was elicited in 29 (29.5%) masses.
Our results were inconsistent with the results of Förnvik et al,11 which stated there was a significant underestimation of the tumour size when compared with the pathology (p < 0.05) for the tested breast imaging modalities, i.e. DM, DBT and ultrasound, although this was least apparent for DBT. An explanation of our findings is that at DBT, the problem of superimposition of a normal glandular tissue on the tumour borders was reduced and so the breast masses were more conspicuous.5
Management options divert between breast carcinomas not just in regard to tumour size but also to multiplicity, which is a very important item in the choice of the surgery.
CESM showed the highest sensitivity among the used modalities of 92.3% in the detection of multiplicity and showed a statistically significant association (p-value of <0.005). On the other hand, CESM presented the highest number of false-positive masses (7.1%, n = 7) and consequently the lowest specificity of 91.7%. The assessment in this modality is based on the detection of abnormal contrast uptake in the breast, which is elicited by the new angiogenesis of the tumours and this sometimes presents an overlap between benign and malignant pathologies, not to mention that contrast-enhanced mammography is an X-ray technique that cannot provide specific soft-tissue characterization; this may be the reason for such low specificity of the contrast mammography. In a study performed by Kamal et al,12 they analyzed the morphology and enhancement characteristics of breast lesions on CESM. They stated that contrast mammography elicited a large number of false-positive cases and recommended MRI for better differentiation of breast tumours.
In 2008,5 a previous study evaluated the performance of DBT and DM in the detection and evaluation of breast calcifications and proved that the DBT was the modality for defining distribution and DM was the one for proper characterization of the different shapes. In our research, the main scope was not the detection, rather than finding an association with distortion in the breast tomosynthesis images and/or abnormal contrast uptake in the contrast-enhanced mammography, since it is common to find microcalcific clusters of punctuate pattern (benign-looking pattern) and not the “pleomorphic”-specific pattern of malignant microcalcifications.
We considered clustered microcalcifications with distortion or contrast uptake as a part of the disease extent. To our knowledge, this point was not mentioned previously in the literature.
In our work, both DBT and DM showed 100% sensitivity, specificity and accuracy in estimating calcifications.
As regards the “T stage” of the breast carcinoma, CESM showed the highest concordant proportion of carcinomas that were staged correctly (n = 80; 79.6%) in correlation with the pathology results. These findings were consistent with Dromain et al,13 who stated that the lesion size by CESM was closer to the histological size as compared with that by DM. They also stated that CESM results showed a significant difference (p-value of 0.002) when compared with DM results while that compared with DBT was statistically insignificant (p-value of 0.1).
As mentioned earlier, we had performed combined evaluation of the performance of DBT and CESM with each other and with the traditional DM and finally the overall performance of DM in addition to DBT and CESM applications in staging of breast carcinomas was also evaluated.
In case of combining only two mammographic modalities, the best merging was between DBT and CESM that showed the highest sensitivities in detection of lesions size (76.8%) and multiplicity (92.3%), with higher specificity (97.4%) due to the lower number of false-positive cases and 100% sensitivity and specificity in the detection of malignant calcifications.
Although the combined evaluation of DM and DBT showed the least sensitivity (n = 72, 75%) for size estimation, it was higher than the value elicited by the performance of each one of them, i.e. DM = 60.2% (n = 59) and DBT = 70.4% (n = 69).
Moreover, combining all three modalities together revealed sensitivity in detection of size (76.8%), multiplicity (92.3%) and calcifications (100%) comparable with that of the combined performance of DBT and CESM.
Previous studies that compared DBT with DM have shown results varying from statistically significant advantage for DBT, such as Svahn et al,10 Smith et al14 and Michell et al,15 to no clear advantage for DBT.16–19 However, other studies suggested improved sensitivity of DBT over DM.5,11
It was mentioned in previous literatures initial experiences with CESM that supports its clinical feasibility.8,10,11,20
Fallenberg et al21 performed a comparative analysis for sizes of breast cancer measured by CESM and contrast-enhanced breast MRI. They found that CESM has a good correlation with post-operative histology in size assessment and that there was no significant difference found between the measurements of the breast tumour on MRI and CESM when compared with histopathology.
Another comparative analysis regarding the size of the breast cancer was performed between CESM and ultrasound in 2014 and they concluded that CESM is accurate in size measurements of small breast tumours. On an average, CESM leads to a slight overestimation of tumour size, whereas ultrasound tends to underestimate tumour size.22
The study had few drawbacks: (1) a limited sample size; (2) the included patients have been exposed to higher doses of radiation than provided by the standard mammogram and in a narrow interval of time, yet this is only for one instance, not on annual basis (like the standard mammogram) and for the sake of optimal staging; (3) not all the cases underwent mastectomy and this gave a chance for missed carcinomas that may be undetectable on imaging (inaccurate false negatives).
CONCLUSION
DBT should be considered as a superior tool to two-dimensional mammography in accurate measurement of the pre-operative tumour size, which is essential for pre-operative planning and neoadjuvant chemotherapy.
CESM may be useful for the “T staging” as well as for the clarification of multicentric and multifocal satellites when used in adjunct to other mammography applications.
Contributor Information
Maha H Helal, Email: drmaha@hotmail.com.
Sahar M Mansour, Email: sahar_mnsr@yahoo.com.
Mai Zaglol, Email: maizaglol@gmail.com.
Lamia A Salaleldin, Email: lamiaadel73@hotmail.com.
Omniya M Nada, Email: omnian44@hotmail.com.
Marwa A Haggag, Email: marwa130140@yahoo.com.
REFERENCES
- 1.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 2002; 225: 165–75. [DOI] [PubMed] [Google Scholar]
- 2.Pisano ED, Yaffe MJ, Hemminger BM. Current status of full-field digital mammography. Acad Radiol 2000; 7: 266–80. doi: https://doi.org/10.1016/S1076-6332(00)80478-X [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg RD, Hunt WC, Williamson MR, Gilliland FD. Effects of age, breast density, ethnicity, and estrogen replacement therapy on screening mammographic sensitivity and cancer stage at diagnosis: review of 183,134 screening mammograms in Albuquerque, New Mexico. Radiology 1998; 209: 511–18. [DOI] [PubMed] [Google Scholar]
- 4.Gur D, Abrams GS, Chough DM, Ganott MA, Hakim CM, Perrin RL, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009; 193: 586–91. doi: https://doi.org/10.2214/AJR.08.2031 [DOI] [PubMed] [Google Scholar]
- 5.Andersson I, Ikeda DM, Zackrisson S, Ruschin M, Svahn T, Timberg P, et al. Breast tomosynthesis and digital mammography: a comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur Radiol 2008; 18: 2817–25. [DOI] [PubMed] [Google Scholar]
- 6.Mercier J, Kwiatkowski F, Abrial C, Boussion V, Dieu-de Fraissinette V, Marraoui W, et al. The role of tomosynthesis in breast cancer staging in 75 patients. Diagn Interv Imaging 2015; 96: 27–35. doi: https://doi.org/10.1016/j.diii.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 7.Luczyńska E, Heinze-Paluchowska S, Dyczek S, Blecharz P, Rys J, Reinfuss M. Contrast-enhanced spectral mammography: comparison with conventional mammography and histopathology in 152 women. Korean J Radiol 2014; 15: 689–96. doi: https://doi.org/10.3348/kjr.2014.15.6.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dromain C, Balleyguier C, Muller S. Evaluation of tumor angiogenesis of breast carcinoma using contrast- enhanced digital mammography. AJR Am J Roentgenol 2006; 187: 528–37. doi: https://doi.org/10.2214/AJR.05.1944 [DOI] [PubMed] [Google Scholar]
- 9.Jong RA, Yaffe MJ, Skarpathiotakis M, Shumak RS, Danjoux NM, Gunesekara A, et al. Contrast-enhanced digital mammography: initial clinical experience. Radiology 2003; 228: 842–50. doi: https://doi.org/10.1148/radiol.2283020961 [DOI] [PubMed] [Google Scholar]
- 10.Svahn T, Chakaborty DP, Ikeda D. Breast tomosynthesis and digital mammography: a comparison of diagnostic accuracy. Br J Radiol 2012; 85: 1074–82. doi: https://doi.org/10.1259/bjr/53282892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Förnvik D, Zackrisson S, Ljungberg O. Breast tomosynthesis: accuracy of tumor measurement compared with digital mammography. Acta Radiol 2010; 51: 240–7. [DOI] [PubMed] [Google Scholar]
- 12.Kamal R, Helal M, Wessam R, Mansour S, Godda I, Alieldin N. Contrast-enhanced spectral mammography: impact of the qualitative morphology descriptors on the diagnosis of breast lesions. Eur J Radiol 2015; 84: 1049–55. [DOI] [PubMed] [Google Scholar]
- 13.Dromain C, Thibault F, Muller S, Rimareix F, Delaloge S, Tardivon A, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol 2011; 21: 565–74. [DOI] [PubMed] [Google Scholar]
- 14.Smith AP, Rafferty EA, Niklason L. Clinical performance of breast tomosynthesis as a function of radiologist experience level. Lect Notes Comput Sci 2008; 5116: 61–6. [Google Scholar]
- 15.Michell MJ, Wasan RK, Iqbal A. Two-view 2D digital mammography versus one-view digital breast tomosynthesis. Breast Cancer Res 2010; 3(Suppl. 12): S1–16. [Google Scholar]
- 16.Poplack SP, Tosteson TD, Kogel CA, Nagy HM. Digital breast tomosynthesis: initial experience in 98 women with abnormal digital screening mammography. AJR Am J Roentgenol 2007; 189: 616–23. [DOI] [PubMed] [Google Scholar]
- 17.Good WF, Abrams GS, Catullo VJ. Digital breast tomosynthesis: a pilot observer study. AJR Am J Roentgenol 2008; 190: 865–9. doi: https://doi.org/10.2214/AJR.07.2841 [DOI] [PubMed] [Google Scholar]
- 18.Teertstra H, Loo C, van den Bosch M. Breast tomosynthesis in clinical practice: initial results. Eur Radiol 2010; 20: 16–24. doi: https://doi.org/10.1007/s00330-009-1523-2 [DOI] [PubMed] [Google Scholar]
- 19.Gennaro G, Toledano A, di Maggio C, Baldan E, Bezzon E, La Grassa M, et al. Digital breast tomosynthesis versus digital mammography: a clinical performance study. Eur Radiol 2010; 20: 1545–53. doi: https://doi.org/10.1007/s00330-009-1699-5 [DOI] [PubMed] [Google Scholar]
- 20.Lewin J, Larke F, Hendrick RE. Dual-energy contrast-enhanced digital subtraction mammography: development and clinical results of a new technique for breast cancer detection. Radiology 2001; 221: 339. [Google Scholar]
- 21.Fallenberg EM, Dromain C, Diekmann F, Engelken F, Krohn M, Singh JM, et al. Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 2014; 24: 256–64. doi: https://doi.org/10.1007/s00330-013-3007-7 [DOI] [PubMed] [Google Scholar]
- 22.Blum KS, Rubbert C, Mathys B, Antoch G, Mohrmann S, Obenauer S. Use of contrast-enhanced spectral mammography for intramammary cancer staging: preliminary results. Acad Radiol 2014; 21: 1363–9. doi: https://doi.org/10.1016/j.acra.2014.06.012 [DOI] [PubMed] [Google Scholar]