Figure 8. GRP75 targeting by MKT-077 enhances G1-phase-privileged uptake of 100 nm nanomicrospheres.
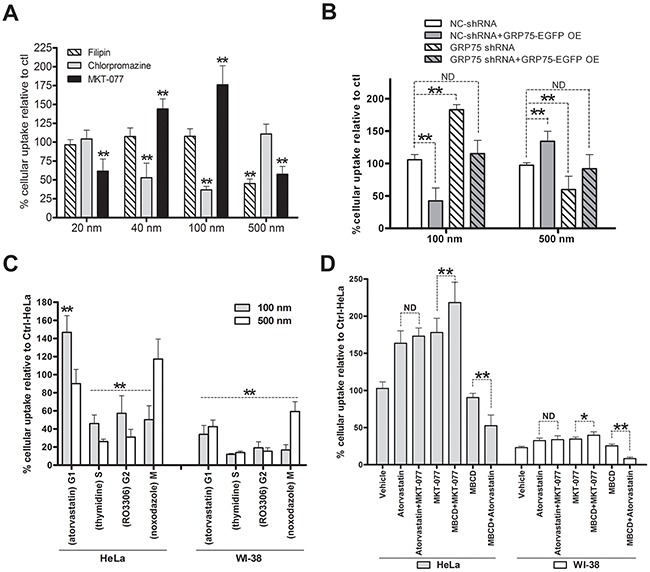
(A) Effect of CME, CvME, and GRP75 inhibitions on the uptake of size-defined nanomicrospheres. HeLa cells were incubated respectively with filipin (5μg/ml), chlorpromazine (10μg/ml), or with MKT-077 (40uM) in serum-free medium for 24h. Subsequently, different sizes of fluorescent microspheres (1:300, v/v) were added and incubated for 12h in the presence of inhibitors. The uptakes of microspheres in cells were determined by flow cytometry analysis. The fluorescent intensity of nanoparticles in DMSO treated cells (Ctrl) was set as 100%. (B) Effect of GRP75 knock-down and overexpression on the uptake of nanomicrospheres. GRP75-shRNA stably transduced HeLa cells (GRP75-shRNA) were transfected with the wtGRP75 plasmid for overexpression (GRP75-OE) for 12h. After extensive washing and continued growth for 18h, 100nm or 500m fluorescent microspheres (1:300) were added to the cells for 12h incubation and processed for flow cytometry analysis. The fluorescent intensity in cells was compared to that of the negative control (NC-shRNA). (C) Effect of arrested cell cycle phases on the uptake of nanomicrospheres. HeLa and WI-38 cells were synchronized at different cell cycle phases as described above. Fluorescent microspheres of 100 and 500nm sizes were respectively added (1:300) and incubated for 12 h in the presence of chemical inducers. The uptakes of microspheres were determined by flow cytometry and intracellular the fluorescent intensity of untreated HeLa cells was set as 100 % (Ctrl-HeLa). (D) Effect of MKT-077 combined with atorvastatin or MBCD on the uptake of 100 nm fluorescent microspheres. HeLa and WI-38 cells were respectively incubated with the mixtures of MKT-077 and atorvastatin, or MKT-077 and MBCD, or MBCD and atorvastatin. The uptakes of nanomicrosphere (1:300) in single or combined treated cells were determined by flow cytometry and compared to that of untreated HeLa cells (Ctrl-HeLa). *0.01 < P < 0.05, **P < 0.01, ND: no difference.
