Fig. 6.
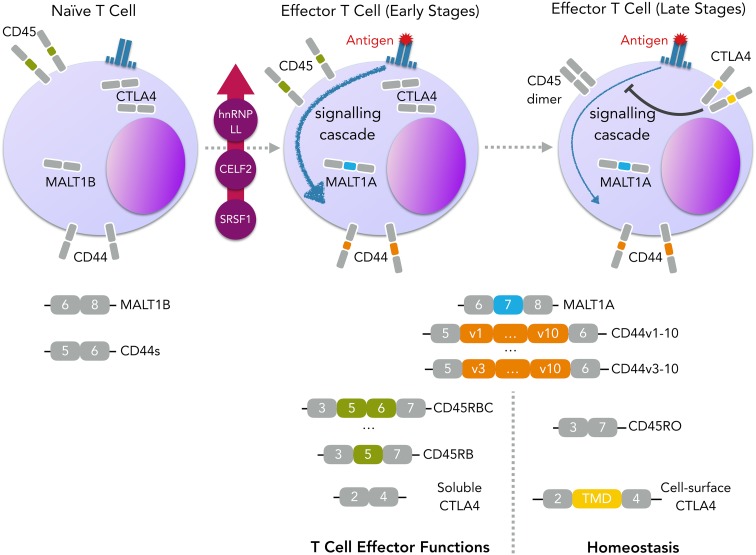
Isoform shifts following T-cell activation. T-cell activation upon antigen recognition leads to global changes in AS, from which the inclusion of MALT1 exon 7 and CD44 variable exons are highlighted. In the case of MALT1, inclusion of TRAF-binding domains contained in its exon 7 leads to a higher recruitment of TRAF6 to the CARMA1-BCL10-MALT1 (CBM) signalling complex, which facilitates IKK activation (Meininger et al. 2016). This results in an enhancement of signalling pathways downstream of TCR signalling and promotion of T-cell activation. As for the transmembrane glycoprotein CD44, ten variable exons are located in the extracellular domain of the protein, which can be excluded or included in different combinations, leading to differences in binding affinity to extracellular matrix components, namely, hyaluronic acid (Naor et al. 1997). While in resting T cells the CD44 variable exons are skipped (isoform CD44s), these are included upon activation (Arch et al. 1992). Even though the importance of this event is not yet clear, CD44 is known to be involved in T-cell homing (DeGrendele et al. 1997) and survival (Baaten et al. 2010). Later stage changes in alternative pre-mRNA splicing often impact genes involved in homeostasis and immunologic memory, from which we take CD45 and CTLA4 as examples. Skipping of alternative exons 4–6 of CD45, results in the production of an isoform more prone to dimerisation, which inhibits the role of CD45 in TCR-signalling transduction. CTLA4, on the other hand, competes with CD28 for ligand binding (van der Merwe et al. 1997), and delivers inhibitory signals that counteract the co-stimulatory signal conferred by CD28 (Krummel and Allison 1995). Upon activation, CTLA4 expression is increased and exon 3, encoding a transmembrane domain, is included (Oaks et al. 2000), drastically increasing the expression of CTLA on the cell surface and empowering the T-cell inhibitory signal. TCR T-cell receptor, TMD transmembrane domain