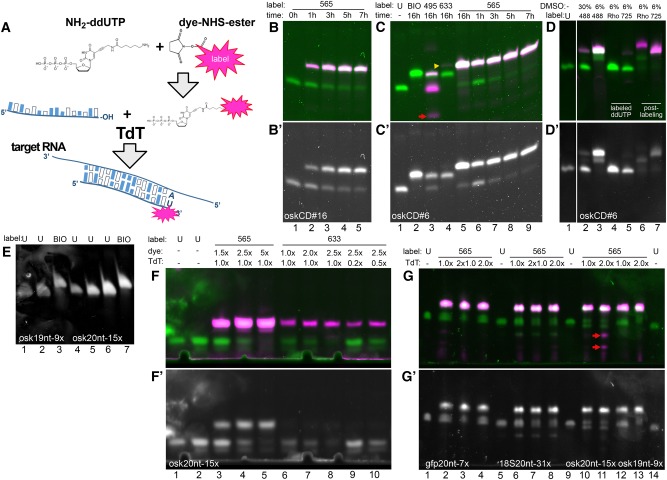
FIGURE 1.
3′ Incorporation of labeled ddUTP by TdT. (A) Schematics of the enzymatic oligo labeling protocol. The volatile dye–NHS ester is reacted with NH2-ddUTP immediately upon reconstitution. The labeled, unpurified terminator nucleotide is then used in a TdT-mediated end-labeling reaction to label the 3′ end of individual oligonucleotides or oligonucleotide mixtures that can serve as smFISH or RNA affinity capture probes in downstream applications. (B,B′) oskCD#16 probe (lane 1) labeled with Atto565–ddUTP (4× molar excess) for 1 h (lane 2), 3 h (lane 3), 5 h (lane 4), and 7 h (lane 5). Dye fluorescence is shown in magenta, SYBR GOLD staining is shown in green (B) and in gray (B′). Ninety-six picomoles of oligo/lane. (C,C′) oskCD#6 probe (lane 1) labeled with biotin–ddUTP (threefold molar excess) (lane 2), Atto495–ddUTP (fourfold molar excess) (lane 3), Atto633–ddUTP (fourfold molar excess) (lane 4), and Atto565–ddUTP (fourfold molar excess) (lane 5) overnight (16 h). Red arrow (lane 3) indicates carryover of free Atto495–ddUTP. Yellow arrowhead points to a minuscule amount of Atto633 labeled, fluorescent product in lane 4. Time course labeling of oskCD#6 probe with Atto565–ddUTP (fourfold molar excess) for 1 h (lane 6), 3 h (lane 7), 5 h (lane 8), and 7 h (lane 9). Ninety-six picomoles of oligo/lane. (D,D′) oskCD#6 probe (lane 1) labeled with Atto488–ddUTP (fourfold molar excess) in the presence of 30% (lane 2) and 6% DMSO (lane 3). oskCD#8 probe labeled directly with AttoRho14–ddUTP (lane 4) and Atto725–ddUTP (lane 5) (fourfold molar excess) or with unconjugated NH2–ddUTP reacted to AttoRho14–NHS ester (lane 6) or Atto725–NHS ester (lane 7) subsequently. Ninety-six picomoles of oligo/lane. (E) osk19nt-9× probe mixture (lane 1, 12 pmol; lane 2, 24 pmol) labeled with biotin–ddUTP (threefold molar excess) (lane 3, 24 pmol). osk20nt-15× probe mixture (lane 4, 6 pmol; lane 5, 12 pmol; lane 6, 24 pmol) labeled with biotin–ddUTP (threefold molar excess) (lane 7, 24 pmol). (F,F′) osk20nt-15× probe mixture (lane 1, 6 pmol; lane 2, 9 pmol) labeled with Atto565–ddUTP (lane 3, 1.5-fold; lane 4, 2.5-fold; lane 5, fivefold molar excess) or with Atto633–ddUTP (lane 6, 1.5-fold; lane 7, twofold; lanes 8–10, 2.5-fold molar excess). Lanes 9 and 10 show results of labeling performed with reduced amounts of TdT enzyme (lane 9, 0.2-fold; lane 10, 0.5-fold of standard TdT amount). Fifteen picomoles of oligo/lane (3–10). (G,G′) Atto565–ddUTP labeling (fivefold excess) of probe mixtures. gfp20nt-7× probe mixture (lane 1) labeled using onefold (lanes 2,3) or twofold (lane 4) the standard TdT amount. 18S20nt-31× probe mixture (lane 5) labeled using onefold (lanes 6,7) or twofold (lane 8) the standard TdT amount. osk20nt-15× probe mixture (lane 9) labeled using onefold (lane 10) or twofold (lane 11) of standard TdT amount. osk19nt-9× probe mixture (lane 14) labeled using onefold (lane 12) or twofold (lane 13) the standard TdT amount. Lanes 3 and 7 show results of relabeling of probe mixtures shown in lanes 2 and 6, respectively. Three picomoles of oligo/lane (1,5,9,14) and 15 pmol oligo/lane (2–4,6–8,10–13). Red arrow (lane 11) indicates carryover of free Atto565–ddUTP.