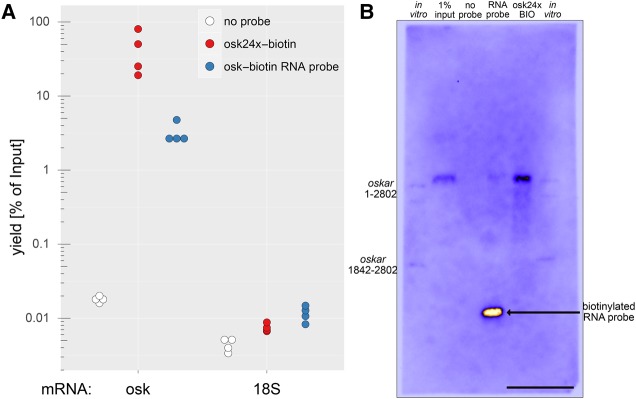
FIGURE 4.
mRNA affinity purification using biotinylated DNA versus RNA probes. (A) Quantitative RT-PCR analysis of the captured RNA. A biotinylated DNA probe set (osk24×–biotin) captured oskar mRNA more efficiently than a single, 400-nt-long, internally biotinylated RNA probe (osk–biotin RNA probe), whereas the unrelated RNA (18S ribosomal RNA) was only marginally captured. (B) Northern blot of the captured oskar mRNA (4 min exposure). Equal volumes of the 1% input (lane 2, 12.5 µg total RNA) and 20%–20% of the three eluates (lanes 3–5) were blotted to a membrane, and oskar mRNA was hybridized with the osk59×–biotin probe set. The signal was detected by HRP-conjugated streptavidin that recognized the biotinylated 400-nt-long RNA probe used for the RNA capture in lane 4. As markers, in vitro transcribed oskar1-2802 (almost full-length RNA, 150 pg/lane) and oskar1842-2802 (almost the entire oskar 3′ UTR, 300 pg/lane) was used (lanes 1,6). Note that oskar1-2802 ran as a doublet, possibly because of two stable RNA conformations. Scale bar is 1 cm.