Figure 1.
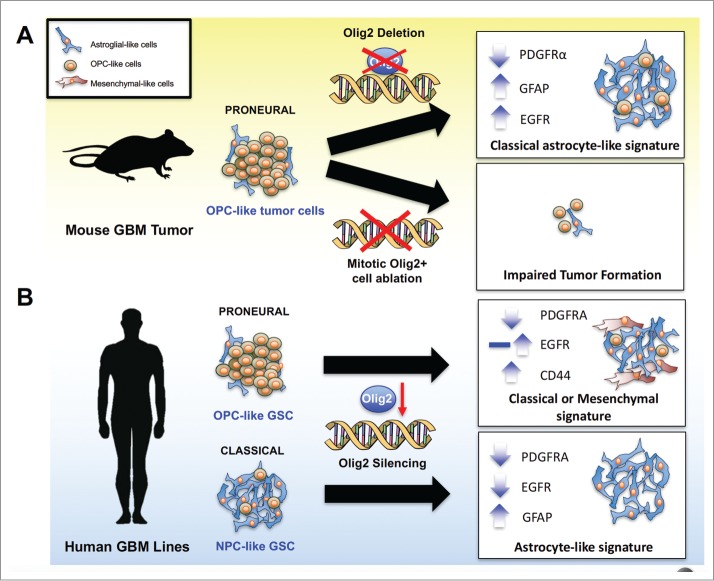
OLIG2+ mitotic cells and OLIG2 function in glioma cell growth (A) In a murine model of proneural GBM, Olig2 knockout delays tumor growth, and changes the gene expression profile from the proneural to the astrocyte-associated classical phenotype, leading to increased EGFR expression and sensitivity to EGFR inhibitors. In addition, the induced suicide of mitotic OLIG2+ cells blocks tumorigenesis, suggesting an essential role of these cells in glioma initiation. (B) In both proneural and classical human GBM cell lines, OLIG2 knockdown downregulates PDGFRA expression, whereas the effect of OLIG2 silencing on full-length EGFR expression differs by genetic background. In proneural OPC-like GSCs, OLIG2 knockdown may exhibit upregulation or no change in the of EGFR expression, resulting in a classical or mesenchymal phenotype shift. In contrast, silencing of OLIG2 in classical NPC-like GSCs leads to EGFR downregulation and GFAP upregulation, manifesting an astrocyte signature.