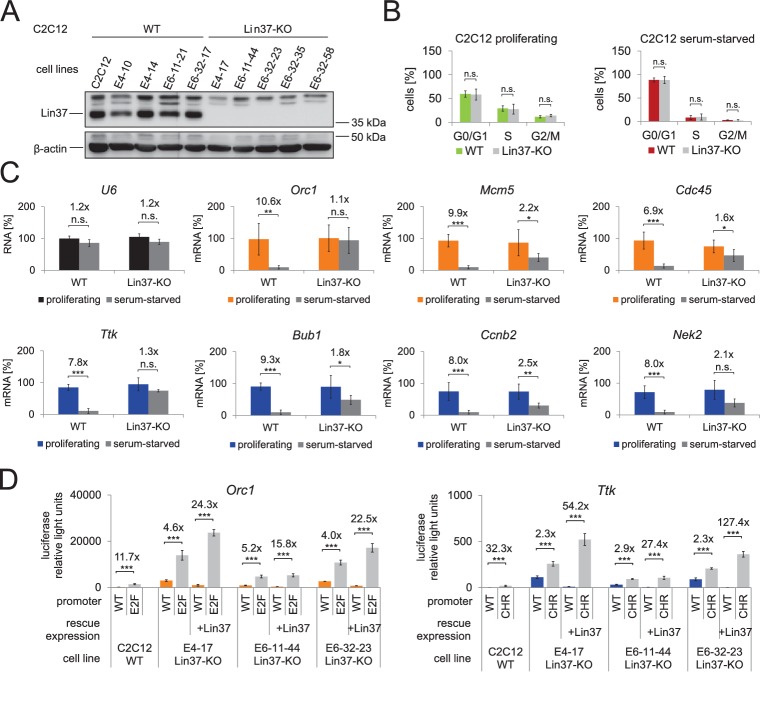
Figure 8. Knockout of Lin37 in C2C12 myoblast cells de-represses cell cycle genes in quiescence.
(A) Western blot analysis of Lin37 wild-type C2C12 parental cells and single cell clones treated with plasmids expressing Cas9 nickase and sgRNAs targeting the 5’ end of Lin37 exon 4 or 6. Cell extracts were tested for expression of Lin37 and β-actin. The five wild-type (WT) and Lin37 knockout (Lin37-KO) lines were utilized for further phenotypic analysis. (B) Lin37 wild-type (WT, n = 5) and Lin37 knockout (Lin37-KO, n = 5) lines were arrested in G0 by serum deprivation. Cell cycle distribution of proliferating and quiescent cells was measured by PI staining and flow cytometry. (C) mRNA expression of G1/S (orange) and G2/M (blue) cell cycle genes was measured by qPCR in all cell lines with two technical replicates each and normalized to U6 expression. All data points for each gene were normalized to the mRNA expression of the proliferating parental cell line which was set to 100%. (D) Serum-starved wild-type (WT) or Lin37 knockout cell lines (Lin37-KO) were transfected with luciferase reporter constructs of the Orc1 (G1/S) and Ttk (G2/M) cell cycle gene promoters. Wild-type (WT) and DREAM-binding site-deficient (E2F, CHR) promoters were tested for their activity. Lin37 knockout was rescued by re-expression of Lin37 (+Lin37). Promoter activities were normalized to Renilla luciferase activity. For all experiments shown in (B), (C), and (D), mean values ± SD are given, and significances were calculated by the Students T-Test (*p≤0.05, **p≤0.01, ***p≤0.001).