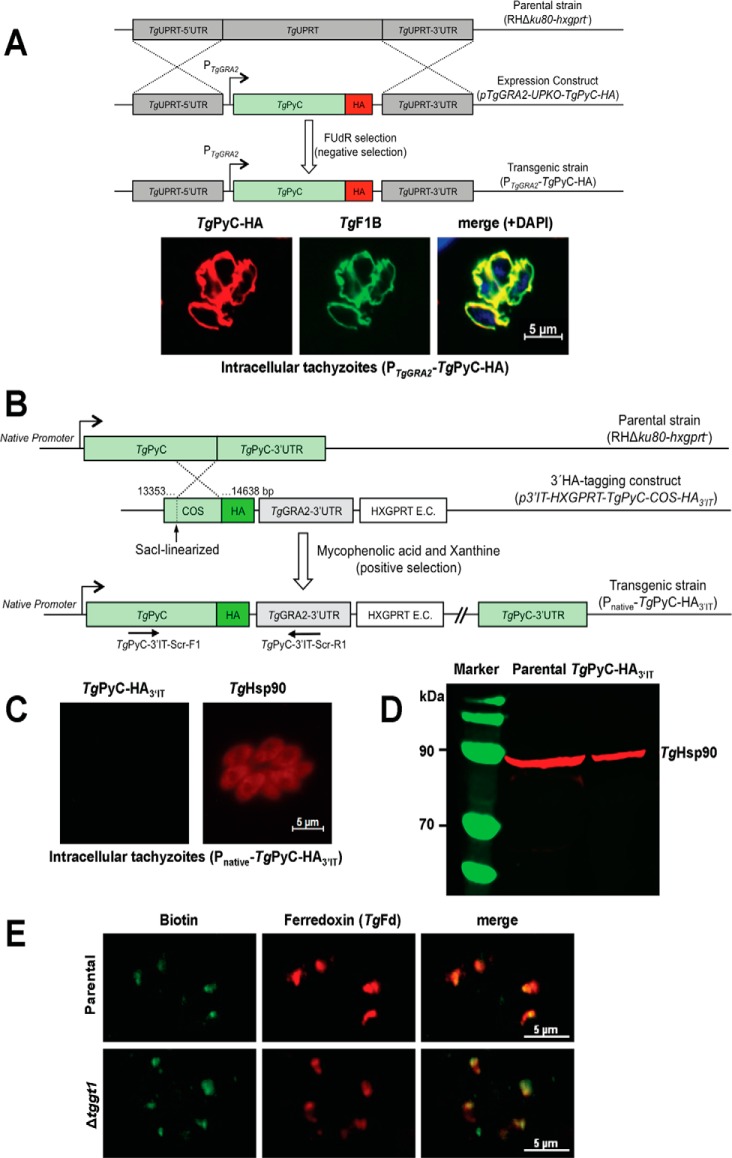
Figure 1.
Ectopically overexpressed pyruvate carboxylase resides in the parasite mitochondrion; however, its endogenous expression is not detectable in the tachyzoite stage of T. gondii. A, schematics of the expression strategy and immunofluorescence images showing subcellular distribution of PyC in tachyzoites. A single copy of TgPyC tagged with HA and regulated by the TgGRA2 elements was targeted at the TgUPRT locus via double homologous recombination in the RHΔku80-hxgprt− strain. Stable transgenic parasites were selected by FUdR for the loss of UPRT function and subjected to immunostaining with α-HA and α-TgF1B antibodies. DAPI staining shows the parasite and host cell nuclei. B, genomic tagging of the endogenous TgPyC with a C-terminal HA epitope in tachyzoites. The construct for 3′-insertional tagging of the TgPyC gene contained a COS and HXGPRT expression cassette (E.C.). It was linearized by SacI enzyme and transfected in the RHΔku80-hxgprt− strain. Parasites expressing TgPyC-HA3′IT under the control of native promoter and TgGRA2 3′-UTR were drug-selected and screened by genomic PCR using recombination-specific primers (TgPyC-3′IT-Scr-F1/R1) and sequencing of the transcript. C and D, detection of TgPyC-HA3′IT by immunofluorescence and Western blot analysis. Intracellular parasites (24-h infection) were immunostained with α-HA and α-TgHsp90 antibodies to monitor the endogenous expression and location of TgPyC-HA3′IT. For immunoblot, extracellular tachyzoites (107) were subjected to SDS-PAGE, blotting, and staining with α-HA and α-TgHsp90 (loading control) antibodies. E, detection of TgPyC in the parental (RHΔku80-hxgprt−) and Δtggt1 strains using α-biotin and α-TgFd antibodies. Note that only acetyl-CoA carboxylase co-localizing with ferredoxin in the apicoplast is visible, which confirms immunodetection of the biotin prosthetic group.