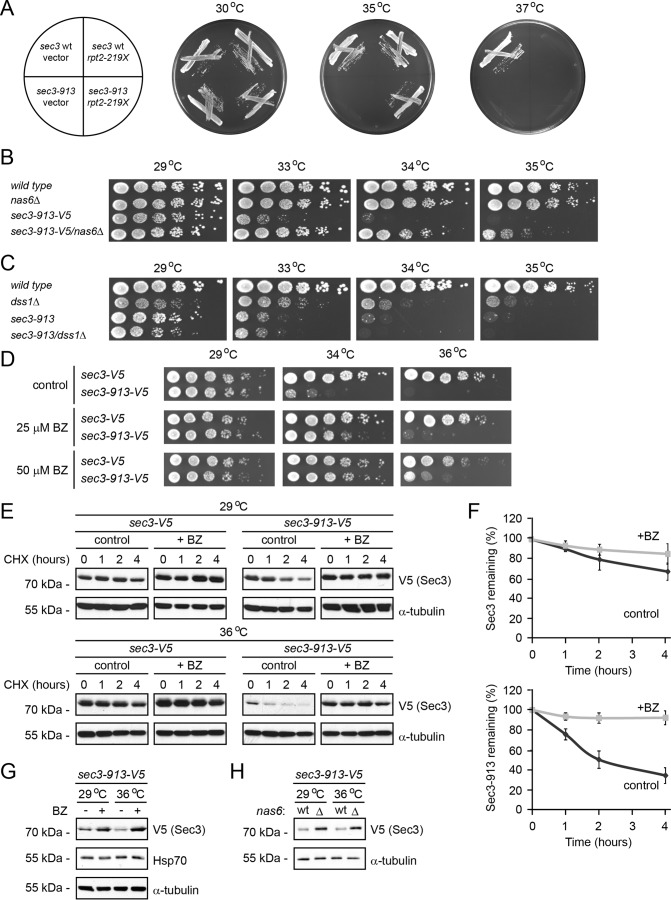
Figure 1.
The Sec3-913 protein is a proteasome substrate. A, wild-type and sec3-913 strains transformed with vector and the rpt2–219X expression construct were streaked onto minimal medium as indicated (left panel) and incubated at 29 °C, 35 °C, and 37 °C. B, growth on rich medium of wild-type, nas6Δ, sec3-913, and the double mutant was compared at the indicated temperatures. C, growth on rich medium of wild-type, dss1Δ, sec3-913, and the double mutant was compared at the indicated temperatures. D, growth on solid medium of wild-type and sec3-913 cells was compared at the indicated temperatures in the absence (control) or presence of 25 or 50 μm BZ. E, degradation of Sec3 and Sec3-913 protein was followed in cultures at 29 °C and 36 °C, where protein synthesis was inhibited with 100 mg/ml CHX for 4 h. To some cultures 1 mm of the proteasome inhibitor BZ was also added. Tubulin served as a control for equal loading. F, quantification of degradation experiments as in E. Top panel, Sec3 (WT) degradation at 36 °C (dark gray, filled diamonds) and Sec3 (WT) at 36 °C with BZ (light gray, filled squares). Bottom panel, Sec3-913 degradation at 36 °C (dark gray, filled diamonds) and Sec3-913 at 36 °C with BZ (light gray, filled squares). The error bars indicate the standard error of the mean (n = 3). G, the steady-state level of Sec3-913 at 29 °C and 36 °C with or without BZ was compared by SDS-PAGE and Western blotting using antibodies to V5 (to detect Sec3-913), Hsp70 and, as a loading control, to α-tubulin. H, the steady-state level of Sec3-913 at 29 °C and 36 °C in wild-type and nas6Δ cells was compared by SDS-PAGE and Western blotting using antibodies to V5 (to detect Sec3-913) and, as a loading control, to α-tubulin.