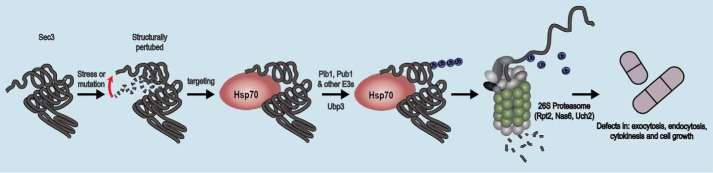
Figure 9.
Model for protein quality control of the exocyst. The data presented here are compatible with a model in which the Sec3 protein, in response to mutations or a stress condition, becomes structurally perturbed, and molecular chaperones detect it as being misfolded. The protein is then ubiquitylated by Pib1 and other E3s, such as Pub1, and directed to the 26S proteasome. At the 26S proteasome, the protein is degraded, leading to loss of function and defects in exocytosis, endocytosis, cytokinesis, and cell growth.