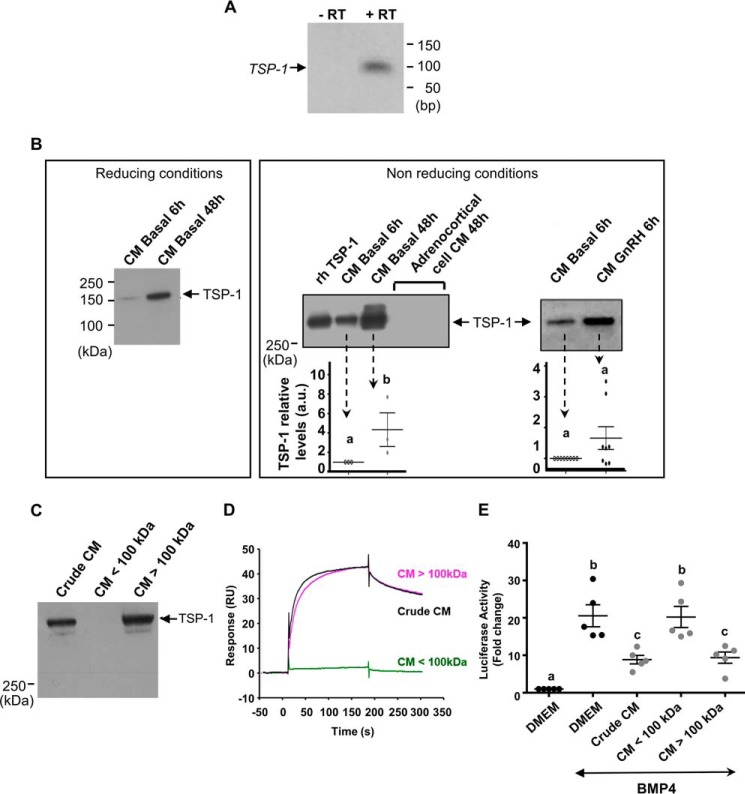
Figure 5.
Expression of pituitary TSP-1 and interaction between TSP-1-enriched CM fractions and BMP-4. A, TSP-1 mRNA expression in ovine pituitary cells. Cells were cultured for 48 h before RNA extraction. TSP-1 mRNA were analyzed by RT-PCR after 30 cycles (lane 2). PCR was performed with omission of reverse transcriptase (lane 1). B, TSP-1 protein expression in CM from pituitary cells. Cells were cultured for 6 h (CM basal 6 h) or 48 h (CM basal 48 h) or 6 h with GnRH (CM GnRH 6 h) before CM recovery and Western blot analysis in reducing or non-reducing conditions. CM from corticoadrenal cells were prepared under the same conditions. As a control, rhTSP-1 (50 ng) was loaded. Protein loading was checked by membrane staining with Ponceau S. The histograms represent the mean ± S.E. of densitometric analysis obtained with CM from six independent pituitary cultures. C, the presence of TSP-1 in high molecular mass fraction from ovine pituitary CM. Media from pituitary cells conditioned for 48 h were fractionated using 100-kDa cut-off membranes. The presence of TSP-1 in the crude CM, the filtrate (CM <100 kDa), and the retentate (CM >100 kDa) was analyzed by Western blotting. Protein loading was checked by membrane staining with Ponceau S. The figure shows a representative experiment. These experiments were performed with CM from six independent pituitary cultures. D, SPR interaction between high molecular mass fraction from pituitary CM and BMP-4. The filtrate (CM <100 kDa) -diluted and the retentate (CM >100 kDa) -diluted obtained as described above were injected for 180 s at 10 μl/min on a 60 RU immobilized BMP-4 flow cell of a CM5 sensor chip. The crude CM diluted 1/10 was injected under the same conditions. The sensorgrams are subtracted with the nonspecific interaction values obtained on the activated-deactivated control flow cell. The figure shows a representative experiment. Independent experiments were performed with CM from three different pituitary cultures. E, antagonization of BMP-4 action by the high molecular mass fraction from pituitary CM on luciferase activity from C3H-B12 cells. C3H-B12 cells were exposed to the crude CM ½-diluted, the filtrate (CM <100 kDa) ½-diluted, and the retentate (CM >100 kDa) -diluted in the presence of rhBMP-4 (2,5 ng/ml) overnight before assaying for luciferase activity. Values are the mean ± S.E. from four independent experiments with duplicate determinations. Bars with different letters indicate that group means are significantly different at p < 0.05.