Figure 1.
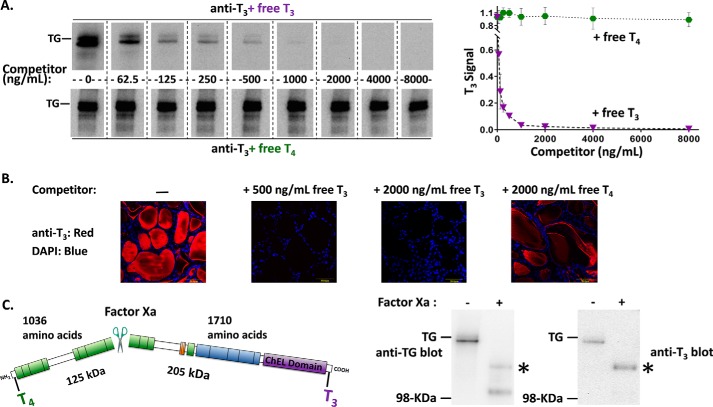
T3 is enriched in the carboxyl-terminal region of TG. A, identical aliquots of normal mouse thyroid lysate (2 μg/lane) were loaded in every other lane, resolved by reducing SDS-PAGE and electrotransfer to nitrocellulose, and the membrane cut into strips. Each membrane strip was incubated with mAb anti-T3 (1:1000) plus an increasing concentration of either free T3 or free T4. The strips were then incubated simultaneously with identical HRP-conjugated goat anti-mouse antibody followed by enhanced chemiluminescence substrate, and then examined together in a single 20-s digital image exposure. Quantitation of the T3 signal within the TG band (gel shown at left; graph shown at right) represents the mean ± S.D. from 3 independent experiments. B, immunofluorescence with the same mAb anti-T3 (1:200) localizes T3-containing protein to the thyroid follicle lumen, and the immunofluorescence signal is specifically blocked upon addition of 500 ng/ml of free T3. All images are the same magnification; the yellow bar in each panel = 50 μm; nuclei are counterstained with DAPI. C, there is a single Factor Xa cleavage site in mouse TG that forms two predicted fragments. TG from normal mouse thyroid lysates ± digestion with Factor Xa was resolved by SDS-PAGE (4 μg of protein per lane), electrotransfer, and immunoblotting with anti-TG or anti-T3 antibodies. Two cleavage products of the expected size were detected with anti-TG; a polypeptide consistent with the C-terminal fragment (asterisk) was enriched in T3. Three independent experiments yielded results identical to those shown here. The position of a pre-stained molecular weight marker is indicated.