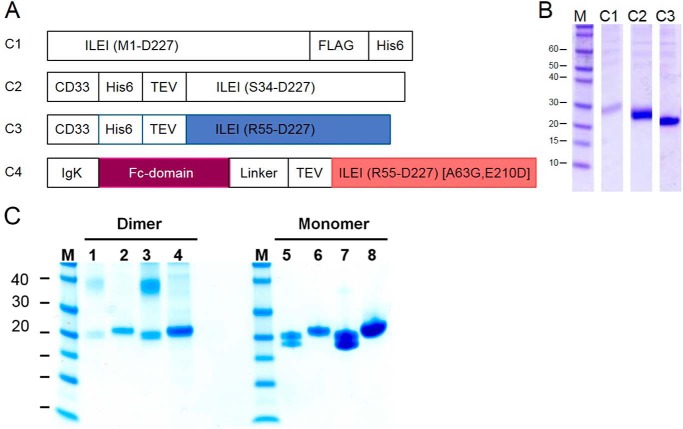
Figure 1.
Constructs for recombinant expression of ILEI in suspension-adapted HEK cells. A, construct 1 containing a native signal peptide and the sequence of human ILEI followed by a FLAG tag and a C-terminal His-tag. Constructs 2 and 3 were designed based on expression of FAM3B PANDER (13) with the native signal peptide replaced by CD33 signal peptide. Human and mouse variants of C3 were used for crystallization experiments. C4 was designed with an N-terminal Fc fusion preceded by an IgK signal peptide. In the C4 construct the ILEI coding region also contains two mutations, Ala-63 mutated to Gly and Glu-210 mutated to Asp. Mouse variants of C3 and C4 were coexpressed to produce a chimeric ILEI heterodimer. B, Coomassie-stained SDS-PAGE showing yields of human ILEI protein in the supernatant after small-scale expression and Ni-NTA purification of constructs hC1, hC2, and hC3. C, SDS-PAGE of mouse ILEI dimer and monomer, construct mC3, under non-reducing (lanes 1, 3, 5, and 7) and reducing conditions (lanes 2, 4, 6, and 8). Samples in lanes 3, 4, 7, and 8 are concentrated versions of lanes 1, 2, 5, and 6. Note the presence of the dimer and the split monomer bands under non-reducing conditions compared with the single band monomer under reducing conditions.